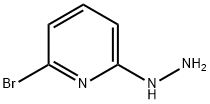
2-BROMO-6-HYDRAZINYLPYRIDINE synthesis
- Product Name:2-BROMO-6-HYDRAZINYLPYRIDINE
- CAS Number:26944-71-8
- Molecular formula:C5H6BrN3
- Molecular Weight:188.03
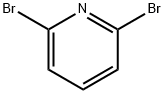
626-05-1

26944-71-8
General procedure for the synthesis of 2-bromo-6-hydrazinopyridine from 2,6-dibromopyridine: commercially available 2,6-dibromopyridine (4.12 g, 16.6 mmol) was suspended in ethanol (40 mL), followed by the addition of hydrazine hydrate (10 mL, 97.6 mmol, 50-60% aqueous solution). The reaction mixture was heated and refluxed in a sand bath at 115°C for 18 hours. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure and the resulting residue was purified by silica gel column chromatography with the eluent ethyl acetate/n-heptane (60/40, v/v) to afford 2-bromo-6-hydrazinopyridine as an off-white solid (3.05 g, 93% yield).1H-NMR (400 MHz, CDCl3) δ: 7.33 (t, 1H), 6.83 (d, 1H ), 6.67 (d, 1H), 6.00 (br-s, 1H), 3.00-3.33 (br-s, 2H).

626-05-1
506 suppliers
$9.00/10g

26944-71-8
158 suppliers
$5.00/1g
Yield:26944-71-8 93%
Reaction Conditions:
with hydrazine hydrate in ethanol;water at 115; for 18 h;
Steps:
11.A
Commercially available 2,6-dibromopyridine (4.12 g, 16.6 mmol) was suspended in ethanol (40 mL) and hydrazine hydrate (10 mL, 97.6 mmol) in water (50-60%) was added. The mixture was heated in a sand-bath at 115° C. for 18 h. The solvent was removed and the residue was purified by chromatography on silica using ethyl acetate/n-heptane (60/40) to afford the title compound as an off-white solid (3.05 g, 93%).1H-NMR (400 MHz, CDCl3): =7.33 (t, 1H), 6.83 (d, 1H), 6.67 (d, 1H), 6.00 (br-s, 1H), 3.00-3.33 (br-s, 2H).
References:
AC Immune, S.A. US2011/256064, 2011, A1 Location in patent:Page/Page column 22
144100-07-2
243 suppliers
$10.00/5g
60-34-4
0 suppliers
$51.79/25g

26944-71-8
158 suppliers
$5.00/1g

144100-07-2
243 suppliers
$10.00/5g

26944-71-8
158 suppliers
$5.00/1g