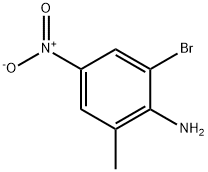
2-BROMO-6-METHYL-4-NITROANILINE synthesis
- Product Name:2-BROMO-6-METHYL-4-NITROANILINE
- CAS Number:102170-56-9
- Molecular formula:C7H7BrN2O2
- Molecular Weight:231.05
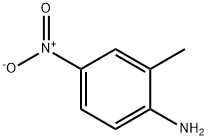
99-52-5

102170-56-9
General procedure for the synthesis of 2-bromo-6-methyl-4-nitroaniline from 4-nitro-2-methylaniline: 2-methyl-4-nitroaniline (2.0 g, 13.2 mmol) was dissolved in acetonitrile (40 mL) at 60 °C, followed by the addition of N-bromosuccinimide (2.8 g, 15.8 mmol). The reaction mixture was heated to reflux for 3 hours and then cooled to room temperature. The reaction mixture was concentrated and diluted with dichloromethane (50 mL). The organic phase was washed sequentially with 2.5 M sodium hydroxide solution and saturated saline, dried over anhydrous sodium sulfate, filtered and concentrated. The crude product was purified by silica gel column chromatography (eluent: n-hexane/ethyl acetate, 4/1, v/v) to afford 2-bromo-6-methyl-4-nitroaniline as a yellow solid (2.75 g, 91% yield). Melting point: 178-179°C. Thin layer chromatography Rf value: 0.40 (unfolding agent: n-hexane/ethyl acetate, 5/1, v/v).1H NMR (DMSO-d6, 400MHz) δ 8.15 (d, J=2.5Hz, 1H), 7.93 (d, J=2.1Hz, 1H), 6.53 (s, 2H), 2.23 (s, 3H). Mass spectrum (ESI) m/z: 228.5 [M-H]-.

99-52-5
285 suppliers
$5.00/5G

102170-56-9
96 suppliers
$7.00/1g
Yield:102170-56-9 99%
Reaction Conditions:
with bromine;acetic acid at 20; for 1.16667 h;
Steps:
A Step A: 2-Bromo-6-rnethyl-4-nitroaniiine.
To a suspension of 2-methyl-4-nitroaniline (10.0 g, 65.7mrnol) in glacial acetic acid (100 rnL) at 20 °C was added bromine (3.4 mL, 66 rnmoi) dropwiseover 40 mm. The mixture was stirred at 20 °C for another 30 mm. Then water (100 rnL) was added, and the resulting precipitate was collected by filtration and dried in vaeuo at 80 °C for 6 h to yield the title compound (14.1 g, 99%) as a yellow solid. MS (ESi): mass calcd. for C7HBrN2O2 23 0.0, m/z found 231 [M+Hft. ‘H NMR (300 MHz, DMSO-d6) ? 8.13 (d, J = 2.2 Hz, iH), 7.89 (d, J= 1.5 Hz, IH), 6.48 (s, 2H), 2.22 (s, 3H).
References:
WO2016/176460,2016,A1 Location in patent:Page/Page column 97
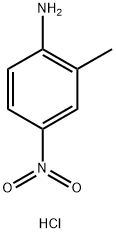
139291-81-9
0 suppliers
inquiry

102170-56-9
96 suppliers
$7.00/1g
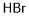
12258-64-9
0 suppliers
inquiry
7722-84-1
0 suppliers
$24.46/*2x50ml

99-52-5
285 suppliers
$5.00/5G

102170-56-9
96 suppliers
$7.00/1g