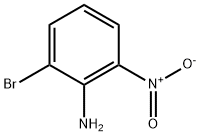
2-BROMO-6-NITROANILINE synthesis
- Product Name:2-BROMO-6-NITROANILINE
- CAS Number:59255-95-7
- Molecular formula:C6H5BrN2O2
- Molecular Weight:217.02
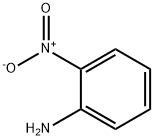
88-74-4

59255-95-7
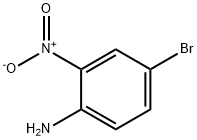
875-51-4
N-bromosuccinimide (44.5 g, 0.25 mol) was added to a solution of 2-nitroaniline (34.5 g, 0.25 mol) in acetic acid (400 mL) in batches over a period of 30 minutes over a temperature range of 308-318 K. The reaction mixture was stirred continuously for 3 hours. The reaction mixture was stirred continuously at 318 K for 3 hours, followed by warming to 363 K and continued stirring for 2 hours. After completion of the reaction, the mixture was cooled to room temperature and poured into vigorously stirred cold water (4 L). After standing for 10 min, the orange precipitate was collected by filtration and washed with cold water (2 x 200 mL). The crude product was recrystallized by 80% ethanol and dried under vacuum to give pure 4-bromo-2-nitroaniline (39.9 g, 74% yield) as an orange crystalline solid. A second crystalline product (6.81 g) was isolated from the mother liquor and analyzed as a mixture of 4-bromo-2-nitroaniline and 2-bromo-6-nitroaniline (ratio 1:0.3). The nuclear magnetic resonance hydrogen spectroscopy (1H NMR) data were in agreement with those reported in the literature (Manley et al., 2003; Lemaire et al., 1989).The 1H NMR (300 MHz, CDCl3) data for 4-bromo-2-nitroaniline: δ 8.26 (dd, J = 2.3, 0.4 Hz, 1H), 7.42 (dd, J = 8.9 , 2.3 Hz, 1H), 6.73 (dd, J = 8.9, 0.4 Hz, 1H), 6.10 (s, 2H).1H NMR (400 MHz, CDCl3) data for 2-bromo-6-nitroaniline: δ 8.14 (dd, J = 8.7, 1.5 Hz, 1H), 7.70 (dd, J = 7.7, 1.5 Hz, 1H), 6.63 (s, 2H). 6.63 (s, 2H), 6.62 (dd, J = 8.7, 7.7 Hz, 1H). Single crystals of 4-bromo-2-nitroaniline and 2-bromo-6-nitroaniline were obtained by sublimation of the second crystallization product (from chloroform) and slow evaporation under reduced pressure conditions (323 K, 10 mbar; 1 bar = 105 Pa), respectively.
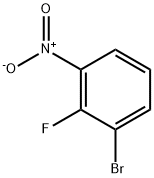
58534-94-4
189 suppliers
$10.00/1g

59255-95-7
138 suppliers
$28.00/1g
Yield:59255-95-7 91.3%
Reaction Conditions:
with ammonia in methanol at 100; for 16 h;Sealed tube;
Steps:
37
2-Bromo-6-nitroaniline A mixture of 1-bromo-2-fluoro-3-nitrobenzene (6.0 g, 27.27 mmol) and NH3 in CH3OH (7 M, 20 mL) was stirred in a sealed tube at 100 °C for 16 h. The solvent was removed and the residue was dissolved in H2O, and then extracted with ethyl acetate. The organic layer was washed with brine, dried over Na2SO4 and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:100) to afford the product as a yellow solid (5.4 g, 91.3% yield).
References:
ARAXES PHARMA LLC;LI, Liansheng;FENG, Jun;WU, Tao;REN, Pingda;LIU, Yi;LIU, Yuan;LONG, Yun, Oliver WO2015/54572, 2015, A1 Location in patent:Page/Page column 271
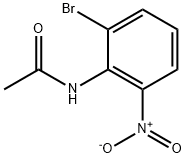
245115-83-7
10 suppliers
$156.80/1g

59255-95-7
138 suppliers
$28.00/1g
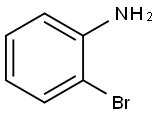
615-36-1
416 suppliers
$10.00/5g

59255-95-7
138 suppliers
$28.00/1g
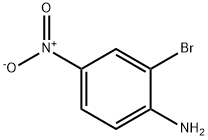
13296-94-1
178 suppliers
$6.00/1g

88-74-4
59 suppliers
$10.00/1g

59255-95-7
138 suppliers
$28.00/1g

875-51-4
305 suppliers
$5.00/1g
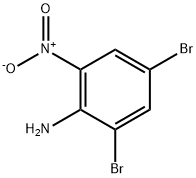
827-23-6
117 suppliers
$15.00/1g