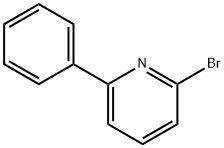
2-Bromo-6-phenylpyridine synthesis
- Product Name:2-Bromo-6-phenylpyridine
- CAS Number:39774-26-0
- Molecular formula:C11H8BrN
- Molecular Weight:234.09
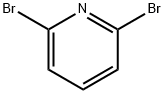
626-05-1
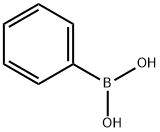
98-80-6

39774-26-0
General procedure for the synthesis of 2-bromo-6-phenylpyridine from 2,6-dibromopyridine and phenylboronic acid: 2,6-dibromopyridine (2 g, 8.44 mmol), phenylboronic acid (1.03 g, 8.44 mmol), potassium carbonate (4.67 g, 33.77 mmol), and tetrabutylammonium bromide (TBAB, 271 mg, 0.84 mmol) were mixed in a 1 ,4-dioxane (16 ml) and water (4 ml) in a solvent mixture. The reaction mixture was degassed by blowing in nitrogen for 30 min, followed by addition of dichlorobis(triphenylphosphine)palladium(II) (PdCl2(dppf), 62 mg, 0.084 mmol). The reaction mixture was stirred at 40 °C for 1 h. After completion of the reaction, the reaction was filtered through diatomaceous earth. The filtrate was collected and the solvent was removed by distillation under reduced pressure. The crude product was purified by column chromatography using 100% petroleum ether as eluent to afford the target compound 2-bromo-6-phenylpyridine as a colorless solid (1.85 g, 93% yield).1H NMR (400 MHz, DMSO) δ 8.56 (dd, J = 6.3,2.4 Hz, 1H), 8.36-8.18 (m, 2H). 8.16-8.00 (m, 2H), 7.61-7.53 (m, 2H), 7.53-7.45 (m, 1H).

626-05-1
508 suppliers
$9.00/10g

98-80-6
743 suppliers
$5.00/5g

39774-26-0
116 suppliers
$41.00/250mg
3558-69-8
114 suppliers
$68.00/5g
Yield: 80%
Reaction Conditions:
with (+/-)-7-(2,3-diacetoxypropyl)theophylline;palladium diacetate;lithium carbonate in water at 120; for 0.166667 h;Microwave irradiation;Green chemistry;Suzuki-Miyaura Coupling;
Steps:
Suzuki-Miyaura couplings were performed in glass tubes suitable for
General procedure: Suzuki-Miyaura couplings were performed in glass tubes suitable for microwave. Halopyridines (0.44 mmol), phenyl boronic acid (65.17 mg, 0.53 mmol), Li2CO3 (63.7 mg, 0.89 mmol), 1 mol % of Pd(OAc)2 (1 mg, 4.45 ϰ 10-3 mol), and 2 mol % of the corresponding ligand in 3 mL of distilled water. The mixtures were stirred and heated at 120 °C under microwave radiation during 10 min with a ramp of 1 min in a CEM Discover reactor coupled to a CEM Explorer robotic system. The resulting reaction mixture was cooled to room temperature and the mixture extracted with CH2Cl2 (3 ϰ 2 mL), the organic phase was treated with anhydrous Na2SO4 after filter over celite and analyzed by Gas Chromatography (GC-MS) on an Agilent 6890N GC with a 30.0 m DB-1MS capillary column coupled to an Agilent 5973 Inert Mass Selective detector. Additional experiments of catalysis were carried out under the same reaction conditions using different bases Na2CO3, K2CO3, Li2CO3, Cs2CO3, Rb2CO3, NaOH, KOH, Et3N, and DIPEA.
References:
Conelly-Espinosa, Patricia;Toscano, Ruben A.;Morales-Morales, David [Tetrahedron Letters,2014,vol. 55,# 42,p. 5841 - 5845]
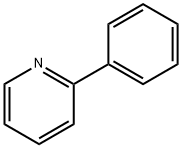
1008-89-5
288 suppliers
$7.00/5g

39774-26-0
116 suppliers
$41.00/250mg

626-05-1
508 suppliers
$9.00/10g

98-80-6
743 suppliers
$5.00/5g

39774-26-0
116 suppliers
$41.00/250mg
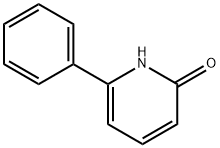
19006-82-7
62 suppliers
$45.00/50mg

39774-26-0
116 suppliers
$41.00/250mg

626-05-1
508 suppliers
$9.00/10g
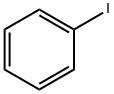
591-50-4
504 suppliers
$10.00/1g

39774-26-0
116 suppliers
$41.00/250mg