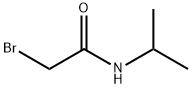
2-bromo-N-isopropylacetamide synthesis
- Product Name:2-bromo-N-isopropylacetamide
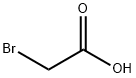
- CAS Number:75726-96-4
- Molecular formula:C5H10BrNO
- Molecular Weight:180.04
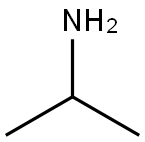
75-31-0
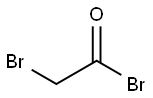
598-21-0

75726-96-4
Dichloromethane (5 mL) and isopropylamine (1.2 mmol) were added to a 25 mL round-bottomed flask, corked tightly and protected under a nitrogen atmosphere. Bromoacetyl bromide (1 mmol) was slowly added dropwise over 2 min under ice bath cooling and stirring conditions. After the dropwise addition, the reaction was continued in an ice bath for 5 min, after which the reaction mixture was transferred to room temperature and stirred for 1 hr. The progress of the reaction was monitored by thin layer chromatography (TLC) until the complete disappearance of bromoacetyl bromide. Upon completion of the reaction, the resulting white solid was collected by filtration and washed with dichloromethane. The filtrate and washings were combined, and the organic layer was sequentially extracted with dilute hydrochloric acid solution, dichloromethane, washed with saturated brine and dried with anhydrous Na2SO4. After concentration under reduced pressure to remove the solvent, the target compound 2-bromo-N-isopropylacetamide was purified by column chromatography to afford the target compound as a white solid in 71% yield.

75-31-0
444 suppliers
$14.00/25mL

598-21-0
312 suppliers
$10.00/10g

75726-96-4
123 suppliers
$50.00/1g
Yield:75726-96-4 71%
Reaction Conditions:
in dichloromethane at 20; for 1 h;Inert atmosphere;Cooling with ice;Concentration;
Steps:
1.1 Preparation of 2-bromo-N-isopropylacetamide I
(5 mL) and isopropylamine (1.2 mmol) were added in a 25 mL round bottom flask, the rubber stopper was plugged and protected with nitrogen. Bromoacetyl bromide (1 mmol) was added over 2 minutes with stirring in an ice- After 5 min, the mixture was stirred at room temperature for 1 h and then reacted by TLC until the bromoacetyl bromide disappeared. After stirring, the white solid was filtered off and washed with dichloromethane. The solution was washed with dilute hydrochloric acid solution, Methyl chloride extraction of organic layer, saturated brine washing, anhydrous Na2SO4 dry, spin dry,Column chromatography gave 2-bromo-N-isopropylacetamide I as a white solid (71% yield)
References:
CN106916145,2017,A Location in patent:Paragraph 0017; 0035-0038; 0058; 0066
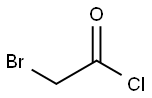
22118-09-8
184 suppliers
$21.21/10gm:

75-31-0
444 suppliers
$14.00/25mL

75726-96-4
123 suppliers
$50.00/1g
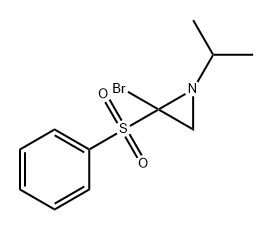
71985-62-1
0 suppliers
inquiry

75726-96-4
123 suppliers
$50.00/1g