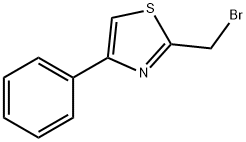
2-(bromomethyl)-4-phenylthiazole synthesis
- Product Name:2-(bromomethyl)-4-phenylthiazole
- CAS Number:78502-79-1
- Molecular formula:C10H8BrNS
- Molecular Weight:254.15
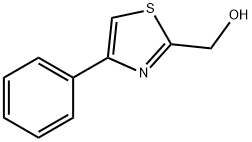
65384-99-8

78502-79-1
General procedure for the synthesis of 2-bromomethyl-4-phenylthiazole from 4-phenyl-2-hydroxymethylthiazole: (4-phenylthiazol-2-yl)methanol (Example 4C, 0.530 g, 2.77 mmol) was dissolved in dichloromethane (10 mL) and cooled to 0°C (ice bath). Phosphorus tribromide (0.118 mL, 1.247 mmol) was added dropwise over 2 min, and a thick white gel formed immediately.After 10 min, the ice bath was removed and the reaction mixture was stirred for 4 h at 22°C. Upon completion of the reaction, the reaction was quenched with ice (~10 g) and the mixture was poured into a mixture of ethyl acetate (150 mL) and saturated sodium bicarbonate solution (50 mL). The organic phase was separated, washed with brine, dried over anhydrous magnesium sulfate and subsequently concentrated under vacuum. The solid residue obtained was purified by silica gel column chromatography (2.5 x 6 cm, toluene as eluent) to afford the target product 2-bromomethyl-4-phenylthiazole (0.561 g, 80% yield) as a light yellow oil, which solidified to a light yellow solid in the refrigerator.LC (Method C): retention time 2.062 min.HRMS (ESI) calculated value C10H9BrSNa [M+Na]+ m/z 253.9634, measured value 253.9655. 1H NMR (CDCl3, 400 MHz) δ ppm: 4.81 (s, 2H), 7.34-7.39 (m, 1H), 7.41-7.47 (m, 2H), 7.52 (s, 1H), 7.86-7.92 (m , 2H).

65384-99-8
26 suppliers
$75.00/100mg

78502-79-1
52 suppliers
$90.00/250mg
Yield:78502-79-1 80%
Reaction Conditions:
with phosphorus tribromide in dichloromethane at 0 - 22; for 4.2 h;
Steps:
4D 2-(Bromomethyl)-4-phenylthiazole
A solution of (4-phenylthiazol-2-yl)methanol (Example 4C, 0.530 g, 2.77 mmol) in dichloromethane (10 mL) was cooled to 0 °C (ice bath) and treated with PBr3 (0.118 mL, 1.247 mmol) added dropwise over 2 min. A heavy white gum was immediately formed. After 10 min, the bath was removed and the solution was stirred at 22 °C for 4 h. The reaction mixture was quenched with ice (~10 g) and poured into a mixture of ethyl acetate (150 mL) and saturated sodium bicarbonate (50 mL). The organic phase was washed with brine, dried over anhydrous magnesium sulfate and concentrated in vacuo. The solid residue was chromatographed on silica gel (2.5 x 6 cm, elution toluene) to give the title material (0.561 g, 80%>) as a light yellow oil which solidified in the fridge to a pale yellow solid. LC (Method C): 2.062 min. HRMS(ESI) calcd for C10H9BrNS [M+H]+ m/z 253.9634, found 253.9655. 1H NMR (CDCl3, 400 MHz) δ ppm: 4.81 (s, 2H), 7.34 - 7.39 (m, 1H), 7.41 - 7.47 (m, 2H), 7.52 (s, 1H), 7.86 - 7.92 (m, 2H).
References:
WO2013/163279,2013,A1 Location in patent:Paragraph 00150
1826-16-0
78 suppliers
$40.00/100mg

78502-79-1
52 suppliers
$90.00/250mg
70-11-1
297 suppliers
$10.00/25g

78502-79-1
52 suppliers
$90.00/250mg

1826-16-0
78 suppliers
$40.00/100mg

78502-79-1
52 suppliers
$90.00/250mg

78502-80-4
0 suppliers
inquiry
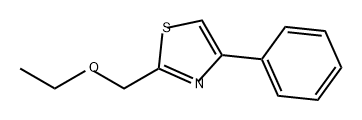
858009-07-1
0 suppliers
inquiry

78502-79-1
52 suppliers
$90.00/250mg