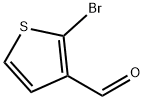
2-BROMOTHIOPHENE-3-CARBALDEHYDE synthesis
- Product Name:2-BROMOTHIOPHENE-3-CARBALDEHYDE
- CAS Number:1860-99-7
- Molecular formula:C5H3BrOS
- Molecular Weight:191.05
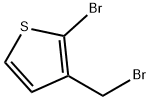
40032-76-6

1860-99-7
The general procedure for the synthesis of 2-bromothiophene-3-carboxaldehyde from 2-bromo-3-bromomethylthiophene is as follows: in a 250-mL two-necked flask, 11.9 g (46 mmol) of undried 2-bromo-3-(bromomethyl)thiophene (molecular weight 256 g/mol) was added. Under nitrogen protection, 20.8 g (74 mmol, 1.6 eq.) of IBX (molecular weight 280 g/mol) and 130 mL of anhydrous DMSO were added to the reaction system.The reaction mixture was heated in an oil bath at 60-70 °C for 3-4 h. The reaction was completed by the addition of 20.8 g (74 mmol, 1.6 eq.) of IBX (molecular weight 280 g/mol) and 130 mL of anhydrous DMSO. Upon completion of the reaction, 20 mL of deionized water was added to quench the reaction. The reaction mixture was extracted with dichloromethane and the precipitate was washed with plenty of dichloromethane to give a white solid. The filtrate was collected by filtration, subsequently dried and purified by column chromatography with the eluent being petroleum ether/ethyl acetate (40:1, V/V).

40032-76-6
125 suppliers
$20.00/250mg

1860-99-7
110 suppliers
$24.00/100mg
Yield:1860-99-7 78.8%
Reaction Conditions:
with 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione in dimethyl sulfoxide at 60 - 70;Inert atmosphere;
Steps:
1.3 3, Preparation of compound 2-bromo-3-thiophenecarboxaldehyde
In a 250 ml two-necked flask, 11.9 g of 2-bromo-3- (bromomethyl) thiophene (256 g mol- 1, 11.9 g, 46 mmol)Pump without water treatment.Under N2 protection, 20.8 g of IBX (280 g mol-1, 20.8 g, 74 mmol, 1.6 equiv)Add 130ml anhydrous DMSO, 60-70°C oil bath for 3-4 hours.After treatment: add 20ml of deionized water to quench the reaction. The mixture was extracted with dichloromethane, washed with a large amount of methylene chloride, and precipitated as a white solid. The filtrate was collected by suction filtration, dried and concentrated by column chromatography.Eluent petroleum ether / ethyl acetate 40: 1 (V: V).
References:
Wuhan University of Technology;Xiao Shengqiang;Chen Run;You Wei;Zhan Chun CN106588868, 2017, A Location in patent:Paragraph 0025; 0034; 0035; 0036; 0037
70260-16-1
64 suppliers
$78.00/250mg

1860-99-7
110 suppliers
$24.00/100mg
616-44-4
253 suppliers
$18.00/25g

1860-99-7
110 suppliers
$24.00/100mg
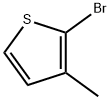
14282-76-9
325 suppliers
$7.00/5g

1860-99-7
110 suppliers
$24.00/100mg
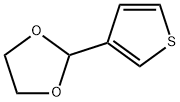
13250-82-3
77 suppliers
$15.00/250mg

1860-99-7
110 suppliers
$24.00/100mg