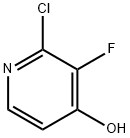
2-Chloro-3-fluoropyridin-4-ol synthesis
- Product Name:2-Chloro-3-fluoropyridin-4-ol
- CAS Number:1184172-46-0
- Molecular formula:C5H3ClFNO
- Molecular Weight:147.53
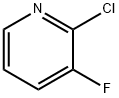
17282-04-1

1184172-46-0
General procedure for the synthesis of 2-chloro-3-fluoro-4-hydroxypyridine: 2-chloro-3-fluoropyridine (2 mmol) was dissolved in anhydrous THF (10 mL) under anhydrous conditions and the solution was cooled to -78 °C. A hexane solution of lithium diisopropylammonium (LDA; 2.2 mmol) was slowly added to this solution at the same temperature. After the reaction mixture was stirred at -78 °C for 2 h, trimethoxyborane (0.48 mL) was added and stirring was continued for 2 h. The reaction mixture was then cooled to -78 °C. Subsequently, peracetic acid (0.72 mL; 32% in dilute acetic acid) was added and the mixture was slowly warmed to 0 °C with stirring and kept for 1 hour. Upon completion of the reaction, the mixture was cooled to 20 °C and aqueous sodium conidiosulfite solution (0.8 g dissolved in 2 mL of water) was added dropwise. The reaction mixture was extracted with ethyl acetate and the organic phases were combined, dried over anhydrous sodium sulfate and concentrated. The residue was purified by silica gel column chromatography with an eluent ratio of 1:19 methanol:dichloromethane to afford the target product 2-chloro-3-fluoro-4-hydroxypyridine as a white solid in 80% yield. The structure of the product was determined by 1H NMR (d6-DMSO): δ 11.86 (br s, 1H, OH), 7.89 (d, J = 5.3 Hz, 1H, C6-H), 6.95 (t, J = 5.8 Hz, 1H, C5-H); 19F NMR (d6-DMSO): δ 141.29 (s); ESI-MS: 148(M + 1)+ Confirmation.

17282-04-1
369 suppliers
$7.00/10g

1184172-46-0
89 suppliers
$45.00/100mg
Yield: 80%
Reaction Conditions:
Stage #1:2-chloro-3-fluoropyridine with lithium diisopropyl amide in tetrahydrofuran;hexane at -78;
Stage #2: with Trimethyl borate in tetrahydrofuran;hexane for 2 h;
Stage #3: with peracetic acid in tetrahydrofuran;hexane;water;acetic acid at 0; for 1 h;
Steps:
9
A solution of 2-chloro-3-fluoropyridine (2 mmol) in anhydrous THF (10ml) was cooled to -780C. To this solution was added a solution of lithium diisopropylamide (LDA; 2.2 mmol) in hexane slowly at same temperature. After 2h at -780C, to the mixture was added trimethoxyborane (0.48ml) and stirred for 2h, followed by an addition of peracetic acid (0.72 ml; 32% in dilute acetic acid). The mixture was allowed to warm to O0C under stirring for Ih, then cooled to -2O0C, sodium dithionite (0.8g in 2ml water) was added dropwise. The mixture was extracted with ethyl acetate and the extract was
References:
BTG INTERNATIONAL LIMITED WO2009/103950, 2009, A1 Location in patent:Page/Page column 22-23