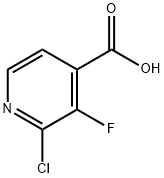
2-Chloro-3-fluoropyridine-4-carboxylic acid synthesis
- Product Name:2-Chloro-3-fluoropyridine-4-carboxylic acid
- CAS Number:628691-93-0
- Molecular formula:C6H3ClFNO2
- Molecular Weight:175.54
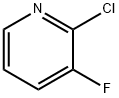
17282-04-1

124-38-9

628691-93-0
1. In a dry reaction flask, lithium diisopropylamide (2M in heptane/tetrahydrofuran/ethylbenzene, 16.7 ml) was mixed with tetrahydrofuran (30 ml) and cooled to -78 °C. 2. A solution of 2-chloro-3-fluoropyridine (4.0 g, 30.4 mmol) in tetrahydrofuran (30 ml) pre-cooled to -78°C was added dropwise to the above cooled mixture over a period of 20 min. 3. After 3 hours of reaction, carbon dioxide was bubbled into the cold reaction mixture for 20 minutes. 4. The reaction mixture was gradually heated to -30 °C for 1 h and then continued to 0 °C. The reaction mixture was heated to -30 °C for 1 h and then continued to 0 °C. 5. The reaction was quenched by addition of water (75 ml) and the aqueous phase was washed with ether (100 ml). 6. The pH of the aqueous phase was adjusted to 2 with 2N hydrochloric acid to produce a white precipitate, which was aged for 18 h. The mixture was filtered and air-dried to give 2-chloro-3-fluoroisonicotinic acid (4.22 g, 79%). 7. 2-Chloro-3-fluoroisonicotinic acid (3.30 g, 18.8 mmol) was suspended in thionyl chloride (40 ml) and heated to reflux for 2.5 hours. 8. The solvent was evaporated and the residue was azeotropically dried with toluene (100 ml) to give a light yellow oil. 9. The oily substance was dissolved in dichloromethane (20 ml), cooled to 0 °C and methanol (2.42 g, 75.3 mmol) was added dropwise over 15 min. 10. After the dropwise addition, the mixture was warmed to room temperature and stirred for 18 hours. 11. The solvent was evaporated and the mixture was partitioned between water (75 ml) and dichloromethane (100 ml). 12. The aqueous phase was further extracted with dichloromethane (100 ml), the organic layers were combined, washed with brine (75 ml), dried over anhydrous sodium sulfate, filtered and evaporated to give methyl 2-chloro-3-fluoroisonicotinate (3.32 g, 93%). 13. 2-Chloro-3-fluoroisonicotinic acid methyl ester (3.32 g, 17.5 mmol) was converted to 8-fluoroimidazo[1,2-a]pyridine-7-carboxylic acid methyl ester (1.4 g, 41%). 14. 8-Fluoroimidazo[1,2-a]pyridine-7-carboxylic acid methyl ester (0.19 g, 1.0 mmol) was brominated to give methyl 3-bromo-8-fluoroimidazo[1,2-a]pyridine-7-carboxylate (195 mg, 71%). 15. 3-Bromo-8-fluoroimidazo[1,2-a]pyridine-7-carboxylic acid methyl ester (0.10 g, 0.37 mmol) was coupled with 4,2'-difluoro-5'-(5,5-dimethyl[1,3,2]dioxaborolan-2-yl)biphenyl-2-ylcarbonitrile (0.15 g, 0.44 mmol) to give 3-(2'-cyano-2,4'-difluoro-biphenyl- 5-yl)-8-fluoroimidazo[1,2-a]pyridine-7-carboxylic acid methyl ester (79 mg, 53%).

17282-04-1
367 suppliers
$7.00/10g

124-38-9
134 suppliers
$175.00/23402

628691-93-0
194 suppliers
$6.00/1g
Yield:628691-93-0 79%
Reaction Conditions:
Stage #1:2-chloro-3-fluoropyridine with lithium diisopropyl amide in tetrahydrofuran;n-heptane;ethylbenzene at -78; for 3.33333 h;
Stage #2:carbon dioxide in tetrahydrofuran;n-heptane;ethylbenzene at 0; for 1.33333 h;
Stage #3: with hydrogenchloride in water; pH=2
Steps:
14 EXAMPLE 14 3-(2'-Cyano-2,4'-difluorobiphenyl-5-yl)-8-fluoroimidazo[1,2-a]pyridine-7- carboxylic acid methyl ester
A mixture of lithium diisopropylamide (2M in heptane/ tetrahydrofuran/ethylbenzene-stabilised with magnesium bis- (diisopropylamide), 16. 7 ml) and tetrahydrofuran (30 ml) was cooled to [- 78°C] and [A PRE-COOLED (-78°C)] solution of 2-chloro-3-fluoropyridine (4.0 g, 30.4 mmol) in tetrahydrofuran (30 ml) was added dropwise over 20 min. After 3 h, carbon dioxide was bubbled through the cold reaction mixture for 20 min. The reaction was then warmed [TO-30°C] for 1 h then warmed [TO 0°C.] The mixture was quenched by the addition of water (75 [ML).] The aqueous phase was washed with diethyl ether (100 ml), then the pH of the solution adjusted to 2 by the addition of 2N hydrochloric acid. The resulting white precipitate was aged for 18 h then filtered and left to air- dry, which afforded [2-CHLORO-3-FLUOROISONICOTINIC] acid as a white solid (4.22 g, 79%): 8H (360 MHz, DMSO) 7. 80 [(1H,] dd, J 5 and 5), 8. 39 [(1H,] d, J 5), 14.20 [(1H,] s). [2-CHLORO-3-FLUOROISONICOTINIC] acid (3.30 g, 18.8 mmol) was suspended in thionyl chloride (40 ml) and heated under reflux for 2.5 h. The solvent was evaporated and the residue dried by means of azeotropic removal of water with toluene (100 ml) to afford a pale yellow oil. The oil was dissolved in dichloromethane (20 ml) and cooled to 0°C before methanol (2.42 g, 75.3 mmol) was added dropwise to the solution over 15 min. On complete addition the mixture was allowed to warm to ambient temperature and stirred for [18] h. The solvent was evaporated and the mixture partitioned between water (75 ml) and dichloromethane (100 ml). The aqueous phase was extracted further with dichloromethane (100 ml), the organic layers were combined, washed with brine (75 ml), dried over anhydrous sodium sulphate, filtered and evaporated to give 2-chloro-3- fluoroisonicotinic acid methyl ester (3.32 g, 93%) as a pale yellow solid: [SN] (360 MHz, [CDCL3)] 3.99 (3H, s), 7.70 [(1H,] dd, J 5 and 5), 8. 31 [(1H,] d, J 5). [2-CHLORO-3-FLUOROISONICOTINIC] acid methyl ester (3.32 g, 17.5 mmol) was converted to 8-fluoroimidazo [[L,] 2-a] pyridine-7-carboxylic acid methyl ester (1.4 g, 41%) as described in Example 1. [8-FLUOROIMIDAZO] [1, 2-a] [PYRIDINE-7-CARBOXYLIC] acid methyl ester (0.19 g, 1.0 mmol) was brominated as described in Example [1,] affording 3- [BROMO-8-FLUOROIMIDAZO] [1, 2-a] pyridine-7-carboxylic acid methyl ester (195 mg, 71%): [8H] (360 MHz, CDCl3) 4.00 (3H, s), 7. 32 [(1H,] t, [J 6.] 9), 7.43 (1H, dd, [J 7] and 6), 8. 19 [(1H,] s), 8. 37 [(1H,] d, [J 7).] 3-Bromo-8-fluoroimidazo [[L,] 2-a] pyridine-7-carboxylic acid methyl ester (0.10 g, 0.37 mmol) and 4, [2'-DIFLUORO-5'- (5,] 5-dimethyl- [1, 3,2] [DIOXABORINAN-2-YL) BIPHENYL-2-CARBONITRILE] (0.15 g, 0.44 mmol) were coupled following the procedure in Example 1 to afford 3- (2'-cyano-2, 4'- difluorobiphenyl-5-yl) -8-fluoroimidazo [1, 2-a] pyridine-7-carboxylic acid methyl ester (79 mg, 53%) as a white solid: [6H] (400 MHz, CDCl3) 4.00 (3H, s), 7.35 [(1H,] dd, J 7 and 7), 7.34-7. 46 (2H, [M), 7. 53-7.] 66 (4H, m), 7.85 (1H, [S),] 8. 24 [(1H,] [D,] J [7)] ; m/z [(ES+)] 408 [(100%,] [MHZ+).]
References:
MERCK SHARP & DOHME LIMITED WO2003/99816, 2003, A1 Location in patent:Page 61