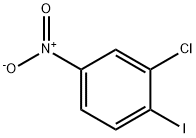
2-chloro-3-iodonitrobenzene synthesis
- Product Name:2-chloro-3-iodonitrobenzene
- CAS Number:683238-59-7
- Molecular formula:C6H3ClINO2
- Molecular Weight:283.45
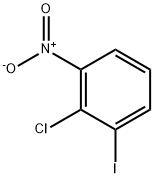
3970-41-0
69 suppliers
inquiry

683238-59-7
21 suppliers
inquiry
Yield:683238-59-7 66%
Reaction Conditions:
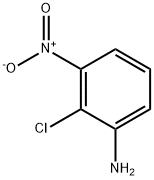
Stage #1: 2-chloro-3-nitroanilinewith hydrogenchloride;sodium nitrite in water at 0 - 5; for 0.5 h;
Stage #2: with potassium iodide in water at 70;Sandmeyer Reaction;
Steps:
Synthetic procedure of compound 5
To an ice-cold solution of 3 (3.46 g, 20 mmol) in 30 mL 37% HCl was dropwise added NaNO2 (1.42g, 20.5mmol) solution with the inner temperature between 0-5°C. After 30 min, a solution of KI (6.64g, 40mmol) in 20 mL H2O was slowly added to the reaction system and then the reaction was gradually warmed to 70 °C and stirred continuously until the gas evolution ceased. Ethyl acetate (3×50 mL) and H2O (100 mL) was added for extraction and the combined organic phase was washed with brine and dried under Na2SO4. The solvent was removed in vacuo and the residue was subjected to column chromatography (eluted by 100% petroleum ether) to afford 5 as a yellow solid. Yield: 66%, m. p. 50-51 °C, 1H NMR (400 MHz, CDCl3) δ 8.09 (d, J = 7.99 Hz, 1H, Ph-H), 7.74 (d, J = 8.04 Hz, 1H, Ph-H), 7.14 (t, J = 7.94 Hz, 1H, Ph-H).
References:
Wei, Wei;Zhou, Shaa;Cheng, Dandan;Li, Yuxin;Liu, Jingbo;Xie, Yongtao;Li, Yonghong;Li, Zhengming [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 15,p. 3365 - 3369] Location in patent:supporting information
99-09-2
20 suppliers
$14.00/1g

683238-59-7
21 suppliers
inquiry
606-22-4
14 suppliers
$58.70/5g

683238-59-7
21 suppliers
inquiry
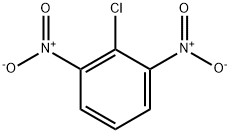
606-21-3
123 suppliers
$45.95/1gm:

683238-59-7
21 suppliers
inquiry
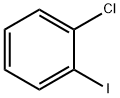
615-41-8
249 suppliers
$5.00/1g
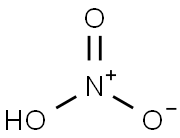
7697-37-2
0 suppliers
$13.00/25g

683238-59-7
21 suppliers
inquiry

32337-97-6
54 suppliers
$18.00/250mg
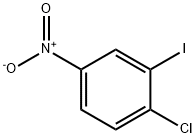
74534-15-9
86 suppliers
$7.00/250mg
41252-96-4
81 suppliers
$24.00/5g