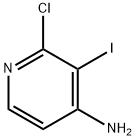
2-CHLORO-3-IODOPYRIDIN-4-AMINE synthesis
- Product Name:2-CHLORO-3-IODOPYRIDIN-4-AMINE
- CAS Number:909036-46-0
- Molecular formula:C5H4ClIN2
- Molecular Weight:254.46
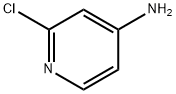
14432-12-3

909036-46-0
General procedure for the synthesis of 2-chloro-3-iodo-4-pyridinamine from 2-chloro-4-aminopyridine: To a 50 mL single-necked round-bottomed flask equipped with a magnetic stir bar, a reflux condenser tube, and a nitrogen inlet tube under nitrogen protection were added 2-chloro-4-aminopyridine (2.50 g, 19.4 mmol, 1.0 equiv.), sodium sulfate trihydrate (3.97 g, 29.2 mmol, 1.5 equiv.), iodine monochloride (3.47 g, 21.4 mmol, 1.1 equiv.) and glacial acetic acid (13.0 mL). 1.5 equiv), iodine monochloride (3.47 g, 21.4 mmol, 1.1 equiv) and glacial acetic acid (13.0 mL). The reaction mixture was heated at 70 °C and magnetically stirred for 16 hours. Complete consumption of the feedstock was confirmed by thin layer chromatography (TLC) and electron bombardment mass spectrometry (GC/MS-EI) analysis. Upon completion of the reaction, the mixture was cooled to room temperature and transferred to a dispensing funnel. Solid sodium bicarbonate (32.3 g) was carefully added in batches to quench the reaction, followed by water (75 mL) and ethyl acetate (75 mL). After complete release of the gas produced, the layers were left to stratify. The aqueous layer was separated and extracted with ethyl acetate (6 x 25 mL). All organic layers were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to remove the solvent. The residue was purified by silica gel column chromatography with hexane/ethyl acetate as eluent (94:6 to 60:40 gradient elution) to afford 2-chloro-3-iodopyridin-4-amine as a light brown solid (2.21 g, 45% yield). The 1H NMR spectrum and low-resolution GC/MS (EI) mass spectrometry data of the product were consistent with those reported in the literature.

14432-12-3
541 suppliers
$5.00/5g
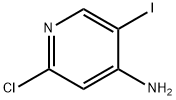
800402-12-4
166 suppliers
$46.00/500mg

909036-46-0
178 suppliers
$31.00/1g
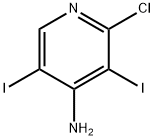
1171919-00-8
55 suppliers
$21.00/100mg
Yield:800402-12-4 49.7%
Reaction Conditions:
with potassium acetate;Iodine monochloride in acetic acid at 70; for 4 h;Inert atmosphere;
Steps:
4.2.1 Experimental procedure for the synthesis of 2-chloro-5-iodopyridin-4-amine (2)
A mixture of 2-chloro-4-amino pyridine 1 (20g, 0.15mol), potassium acetate (22.9g, 0.23mol) and ICl (27.7g, 0.17mol) in glacial acetic acid (200mL) was heated to 70°C for 4h. The solvent was concentrated under reduced pressure. The residue was neutralized with 10% NaHCO3 solution (250mL) and extracted with two 300-mL portions of EtOAc. The combined organic extracts were washed with brine (200mL) and dried over anhydrous Na2SO4. The solvent was removed in vacuum. The crude product showed a mixture of iodopyridines 2, 3 and 4 in the ratio 45:45:10. The required compound 2 (elution 2) was isolated via normal phase preparative HPLC (mobile phase: 60:40, 0.1% TFA in hexane-IPA; column: SunFire Silica 19*150mm, 5μm; Flow rate: 18.0mL/min) in 49.7% yield (19.7g) as an off white solid.
References:
Jose, Gilish;Suresha Kumara;Nagendrappa, Gopalpur;Sowmya;Jasinski, Jerry P.;Millikan, Sean P.;Chandrika;More, Sunil S.;Harish [European Journal of Medicinal Chemistry,2014,vol. 77,p. 288 - 297]

14432-12-3
541 suppliers
$5.00/5g

909036-46-0
178 suppliers
$31.00/1g

14432-12-3
541 suppliers
$5.00/5g

800402-12-4
166 suppliers
$46.00/500mg

909036-46-0
178 suppliers
$31.00/1g