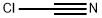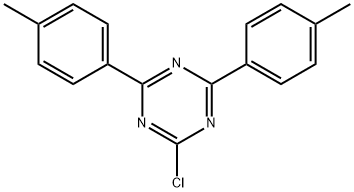
2-chloro-4,6-di-p-tolyl-1,3,5-triazine synthesis
- Product Name:2-chloro-4,6-di-p-tolyl-1,3,5-triazine
- CAS Number:21902-34-1
- Molecular formula:C17H14ClN3
- Molecular Weight:295.77
Yield:21902-34-1 84%
Reaction Conditions:
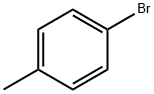
Stage #1:para-bromotoluene with magnesium in tetrahydrofuran for 1.5 h;Reflux;
Stage #2:cyanogen chloride in tetrahydrofuran at 20; for 12 h;
Steps:
1c 2-Chloro-4,6-di-p-tolyl-1,3,5-triazine
2-Chloro-4,6-di-p-tolyl-1,3,5-triazine
7.1 g of magnesium (292 mmol) are initially introduced in a 500 ml four-necked flask, and a solution of 50 g of bromo-p-toluene (292.3 mmol) in 200 ml of THF is slowly added dropwise.
The reaction mixture is heated at the boil for 1.5 hours and subsequently cooled to room temperature.
Cyanogen chloride (21.5 g, 117 mmol) in 200 ml of THF is initially introduced in a second flask and cooled to 0° C.
The cooled Grignard reagent is then added dropwise at this temperature, and the mixture is stirred at RT for 12 h.
After this time, 150 ml of HCl are added to the reaction mixture, and the aqueous phase is extracted three times with dichloromethane.
The combined organic phases are washed with water and dried over Na2SO4 and evaporated.
The residue is recrystallised from EtOH.
The yield is 29.5 g (84%).
References:
Merck Patent GmbH;Pflumm, Christof;Buesing, Arne;Parham, Amir Hossain;Fortte, Rocco;Heil, Holger;Stoessel, Philipp;Mujica-Fernaud, Teresa US2013/53558, 2013, A1 Location in patent:Paragraph 0157; 0158
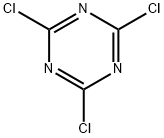
108-77-0
407 suppliers
$13.00/25g
106-38-7
410 suppliers
$14.00/5g

21902-34-1
66 suppliers
$12.00/250mg

108-77-0
407 suppliers
$13.00/25g
4294-57-9
125 suppliers
$50.00/10ml

21902-34-1
66 suppliers
$12.00/250mg