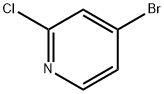
2-Chloro-4-bromopyridine synthesis
- Product Name:2-Chloro-4-bromopyridine
- CAS Number:73583-37-6
- Molecular formula:C5H3BrClN
- Molecular Weight:192.44
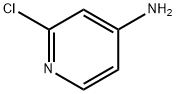
14432-12-3

73583-37-6
Step a: Synthesis of 2-chloro-4-bromopyridine To 8.9 g (69.2 mmol) of 2-chloro-4-aminopyridine was added 81.5 mL of 48% aqueous hydrobromic acid at 0 °C. Subsequently, 33.4 g (208.75 mmol) of bromine monomer was slowly added over 10 min. The reaction mixture was cooled to -10°C and 10.65 g (154 mmol) of sodium nitrite dissolved in 20 mL of water was added dropwise over 30 min. After the dropwise addition was completed, stirring was continued at -10°C for 10 minutes and then brought to room temperature and stirred for 1.5 hours. Upon completion of the reaction, the mixture was cooled to 5°C and saturated aqueous sodium sulfite solution was added until the reaction solution was colorless. The reaction solution was adjusted to alkaline with 35% sodium hydroxide aqueous solution. The basic aqueous phase was extracted twice with diethyl ether, the organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated. The yellow oil obtained (13 g) was purified by silica gel column chromatography (eluent: ethyl acetate/heptane=1/9). The product was a light-colored oily substance in 52% yield. 1H NMR (CDCl3): δ 8.25 (d, 1H, J = 5Hz), 7.55 (s, 1H), 7.4 (d, 1H, J = 5Hz).

14432-12-3
540 suppliers
$5.00/5g

73583-37-6
333 suppliers
$9.00/1g
Yield:73583-37-6 96%
Reaction Conditions:
with tert.-butylnitrite;copper(ll) bromide in acetonitrile at 0 - 20; for 16 h;regioselective reaction;Sandmeyer Reaction;
Steps:
General procedure for the synthesis of substrates
General procedure: To a mixture of copper source (1.2 eq., CuBr2 for 4b, CuCl2 for 4c, CuI for 4d)in acetonitrile (0.4M) was slowly added tBuONO (1.5 eq.). The mixture was stirred for15 min and then cooled to 0°C. A solution of 2-chloro-4-aminopyridine 3 (1.0 eq.) inacetonitrile (0.5M) was slowly added. The mixture was stirred for 1h at 0°C and thenallowed to warm to r.t. for 16h. After concentration in vacuo, aq. 15% ammonia solution (1.3 mL/mmol) was added, and the aqueous layer was extracted withdichloromethane (10 mL/mmol; 3x). The combined organic layers were washed withbrine, dried (Na2SO4), filtered and carefully concentrated under reduced pressure(CAUTION: volatile products) to give the crude 4b-d, which was used in the next stepwithout purification.
References:
Honraedt, Aurélien;Gallagher, Timothy [Synlett,2016,vol. 27,# 1,art. no. ST-2015-D0455-L,p. 67 - 69] Location in patent:supporting information
30766-03-1
271 suppliers
$7.00/1g

73583-37-6
333 suppliers
$9.00/1g
52200-48-3
371 suppliers
$11.00/1g

73583-37-6
333 suppliers
$9.00/1g
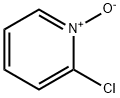
2402-95-1
192 suppliers
$55.00/1g

73583-37-6
333 suppliers
$9.00/1g
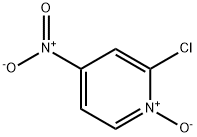
14432-16-7
215 suppliers
$10.00/1g

73583-37-6
333 suppliers
$9.00/1g