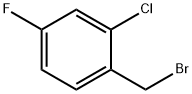
2-Chloro-4-fluorobenzyl bromide synthesis
- Product Name:2-Chloro-4-fluorobenzyl bromide
- CAS Number:45767-66-6
- Molecular formula:C7H5BrClF
- Molecular Weight:223.47
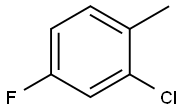
452-73-3

45767-66-6
General procedure for the synthesis of 2-chloro-4-fluorobenzyl bromide from 2-chloro-4-fluorotoluene: A mixture of 77.6 g (0.54 mol) of 2-chloro-4-fluorotoluene, 95.6 g (0.54 mol) of N-bromosuccinimide, 1 g of benzoyl peroxide, and 1,000 mL of carbon tetrachloride was heated and refluxed for 3 hours. After completion of the reaction, the mixture was cooled to room temperature and filtered through a G3 glass sand core funnel. The precipitate was washed with 3 x 100 mL of carbon tetrachloride. The filtrates were combined and concentrated to dryness under reduced pressure. The residue was purified by fractional distillation and the fractions with boiling points of 97-99 °C/10 mmHg were collected to give pure 2-chloro-4-fluorobenzyl bromide in a yield of 106 g (88% yield). Elemental analysis (C7H5BrClF) Calculated values: C, 37.62; H, 2.26; measured values: C, 37.79; H, 2.34. 1H NMR (CDCl3) δ: 7.41 (dt, J = 8.4 Hz, 6.0 Hz, 1H, 6-H), 7.13 (dt, J = 8.4 Hz, 2.6 Hz, 1H, 3-H), and 6.97 (dt, J = 8.4 Hz, 2.6 Hz, 1H, 5-H), 4.55 (s, 2H, CH2).

452-73-3
365 suppliers
$6.00/25g

45767-66-6
208 suppliers
$8.00/10g
Yield:45767-66-6 88%
Reaction Conditions:
with N-Bromosuccinimide;dibenzoyl peroxide in tetrachloromethane; for 3 h;Heating / reflux;
Steps:
12
A mixture of 77.6 g (0.54 mol) of 2-chloro-4-fluorotoluene, 95.6 g(0.54 mol) of N-bromosuccinimide, 1 g of benzoyl peroxide, and 1000 ml of carbon tetrachloride were refluxed for 3 h. The resulting mixture was cooled to room temperature and then filtered through a glass frit (G3). The precipitate was additionally washed with 3 x 100 ml of carbon tetrachloride. The combined filtrate was evaporated to dryness. Fractional distillation of the residue gave pure title product, bp 97-99°C/10 mm Hg. Yield 106 g (88%).Anal. calc. for C7H5BrClF: C, 37.62 H, 2.26 Found: C, 37.79; H, 2.34.1H NMR (CDCl3): δ 7.41 (dd, J=8.4 Hz, J=6.0 Hz, IH, 6-H), 7.13 (dd, J=8.4 Hz, J=2.6 Hz, IH, 3-H), 6.97 (dt, J=8.4 Hz, J=2.6 Hz, IH, 5-H), 4.55 (s, 2H, CH2).
References:
WO2007/70041,2007,A1 Location in patent:Page/Page column 115