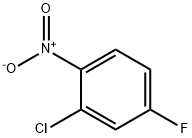
2-Chloro-4-fluoronitrobenzene synthesis
- Product Name:2-Chloro-4-fluoronitrobenzene
- CAS Number:2106-50-5
- Molecular formula:C6H3ClFNO2
- Molecular Weight:175.54
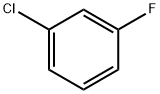
625-98-9

2106-50-5
(a) 3-Chlorofluorobenzene (240 g, 1.85 mol) was slowly added to a mixture of sulfuric acid (185 g, 1.85 mol) and nitric acid (166 g, 1.85 mol) pre-cooled to -5°C. The reaction was carried out at the same temperature for 13 hours. The reaction temperature was maintained at -5°C with continuous stirring for 3.5 hours, followed by continued stirring at the same temperature for 13 hours. Upon completion of the reaction, benzene (200 mL) and hexane (200 mL) were added to the reaction mixture for extraction. The organic phases were combined and washed sequentially with water (300 mL), sodium carbonate solution (300 mL) and water (300 mL). The organic layer was dried over anhydrous sodium sulfate and the solvent was removed by distillation under reduced pressure. The residue was purified by distillation to give 138 g of the mixed isomer. The 4-nitro isomer was isolated from the mixed isomer by crystallization and filtered to give 3-chloro-4-nitrofluorobenzene (51 g, 16.7% yield) with a melting point of 36°C-38°C.

625-98-9
194 suppliers
$14.00/25g

2106-50-5
173 suppliers
$14.00/1g
Yield:2106-50-5 16.7%
Reaction Conditions:
with sulfuric acid;nitric acid in hexane;benzene
Steps:
37.a (a)
(a) 3-Chloro-4-nitrofluorobenzene m-Chlorofluorobenzene (240 g. 1.85 moles) is added to a mixture of sulfuric acid (185 g. 1.85 moles) and nitric acid (166 g., 1.85 moles) at -5° C. in 3.5 hours, stirred 13 hours, then benzene (200 ml.) and hexane (200 ml.) ae added. The extract is washed with water (1+300 ml.), sodium carbonate solution (1*300 ml.), and water (1*300 ml.), dried and the solvents removed. The residue is distilled to give 138 g. of mixed isomers. The 4-nitro isomer crystallizes and is filtered off to give 3-chloro-4-nitrofluorobenzene (51 g. 16.7%) m.p. 36°-38° C.
References:
Rohm and Haas Company US4330324, 1982, A
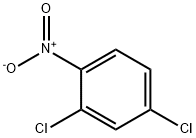
611-06-3
32 suppliers
$15.00/25g

2106-50-5
173 suppliers
$14.00/1g
446-35-5
433 suppliers
$5.00/10g
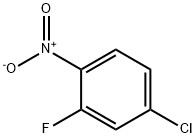
700-37-8
217 suppliers
$9.00/1g
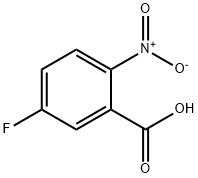
320-98-9
298 suppliers
$8.00/5g

2106-50-5
173 suppliers
$14.00/1g

611-06-3
32 suppliers
$15.00/25g

2106-50-5
173 suppliers
$14.00/1g