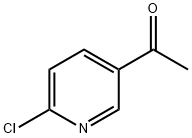
2-Chloro-5-acetylpyridine synthesis
- Product Name:2-Chloro-5-acetylpyridine
- CAS Number:55676-22-7
- Molecular formula:C7H6ClNO
- Molecular Weight:155.58
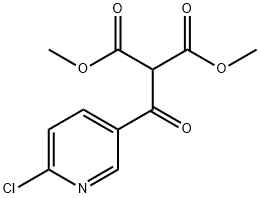
923034-23-5

55676-22-7
Step 3 Synthesis of 1-(6-chloropyridin-3-yl)ethanone: Dimethyl 2-(6-chloronicotinoyl)malonate (89.8 g, 331 mmol) was dissolved in a solvent mixture of DMSO (445 mL) and water (11 mL), and the reaction was heated for 2.5 hr at 130 °C. After the reaction was completed, the reaction mixture was cooled and poured into H2O/ice (300 mL). The aqueous phase was extracted with EtOAc (4 x 150 mL). The organic phases were combined, washed with brine, dried and concentrated under reduced pressure. The residue was recrystallized by 60% ethanol-water solution to afford the target compound 1-(6-chloropyridin-3-yl)ethanone in a yield of 16.5 g (32% yield) as a yellow solid.
53939-30-3
482 suppliers
$5.00/1g
78191-00-1
286 suppliers
$6.00/5g

55676-22-7
270 suppliers
$7.00/100mg
Yield:55676-22-7 84%
Reaction Conditions:
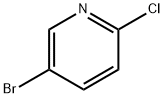
Stage #1: 5-bromo-2-chloropyridinewith TurboGrignard in tetrahydrofuran at 0; for 0.25 h;Inert atmosphere;
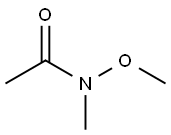
Stage #2: N-Methoxy-N-methylacetamide in tetrahydrofuran at 0 - 20;Inert atmosphere;
Steps:
IM15: 1-(6-Chloro-pyridin-3-yl)-ethanone
A round bottomed flask was charged with 5-bromo-2-chloropyridine (5.30 g, 27.6 mmol) in THF under N2 and cooled at 0° C. A solution of 1 M iso-propylmagnesiumchloride-lithium chloride complex in THF (40 mL) was added drop wise over 15 min. After 70 min N-methoxy-N-methylacetamide (4.1 mL, 38 mmol) was added drop wise. After stirring for 5 min at 0° C. the cooling bath was removed. The mixture was left stirring overnight and was then quenched by the addition of 100 mL saturated aqueous NH4Cl solution. The mixture was extracted with 3×100 mL EtOAc. The combined organic layers were washed with water followed by brine and dried over MgSO4. Evaporation of the volatiles at 80° C., 10 mbar for 1 h gave the title compound (3.596 g, 84) sufficiently pure for the next step.
References:
US2013/12530,2013,A1 Location in patent:Paragraph 0187

923034-23-5
10 suppliers
inquiry

55676-22-7
270 suppliers
$7.00/100mg
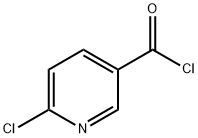
58757-38-3
210 suppliers
$6.00/1g
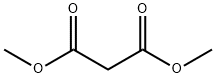
108-59-8
648 suppliers
$5.00/25g

55676-22-7
270 suppliers
$7.00/100mg

58757-38-3
210 suppliers
$6.00/1g

55676-22-7
270 suppliers
$7.00/100mg
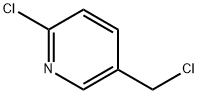
70258-18-3
527 suppliers
$5.00/5g

108-59-8
648 suppliers
$5.00/25g

55676-22-7
270 suppliers
$7.00/100mg