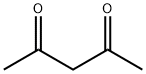
Acetylacetone synthesis
- Product Name:Acetylacetone
- CAS Number:123-54-6
- Molecular formula:C5H8O2
- Molecular Weight:100.12
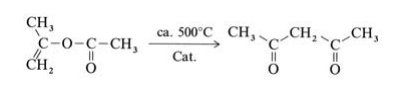
Isopropenyl acetate vapor is fed at atmospheric pressure through a V2A steel tube with an inner temperature of 520℃. The hot reaction gases are quenched, condensed, and cooled to 20℃, whereby the gaseous byproducts carbon monoxide, carbon dioxide, methane, and ketene are separated. The product is purified by fractional distillation. Other industrially less important processes for the production of 2,4-pentanedione, include the Claisen ester condensation of ethyl acetate with acetone using sodium ethoxide as condensation agent and the acetylation of acetoacetic acid esters with acetic anhydride in the presence of magnesium salts.
675-10-5
241 suppliers
$9.00/1g

123-54-6
582 suppliers
$10.00/25ml
Yield:-
Reaction Conditions:
Amberlyst 70 in water at 100; under 22502.3 Torr; for 4 h;Product distribution / selectivity;Inert atmosphere;
Steps:
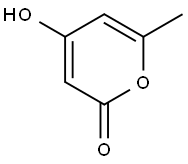
The reactions were carried out in a 50-mL batch reactor, at 100° C., under an inert atmosphere, at elevated pressure (3 MPa). The batch reaction time was 1-16 h. Detailed results are shown in Table 1. An initial reactant mixture of 0.8-1.8 wt % 4-hydroxy-6-methyl-2-pyrone (1, RCH3) dissolved in a solvent was employed. Specifically, the hydration and decarboxylation of 4-hydroxy-6-methyl-2-pyrone (1, RCH3) proceeded over Amberlyst 70-brand catalyst at a mass ratio of 4-hydroxy-6-methyl-2-pyrone:catalyst=2:1. Using water as the solvent, 95% conversion of 4-hydroxy-6-methyl-2-pyrone was achieved with quantitative selectivity to the desired 2,4-pentanedione (2, RCH3) product (Table 1, Entry 2). Under identical reaction conditions using tetrahydrofuran as the solvent, 62% conversion of 4-hydroxy-6-methyl-2-pyrone (1, RCH3) was achieved, with 65% selectivity to the desired 2,4-pentanedione (2, RCH3) product (Table 1, Entry 6). The reaction can also be accomplished in water without the aid of a catalyst (Table 1, Entries 3 and 4), where close to quantitative yields of desired 2,4-pentanedione (2, RCH3) product can be similarly achieved.
References:
US2012/283477,2012,A1 Location in patent:Page/Page column 3-4
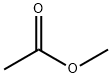
79-20-9
582 suppliers
$14.00/100g
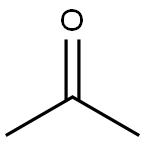
67-64-1
0 suppliers
$19.10/10ml

123-54-6
582 suppliers
$10.00/25ml
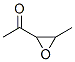
17257-79-3
5 suppliers
inquiry

123-54-6
582 suppliers
$10.00/25ml
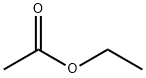
141-78-6
685 suppliers
$22.37/250ml

67-64-1
0 suppliers
$19.10/10ml

123-54-6
582 suppliers
$10.00/25ml

74-86-2
76 suppliers
inquiry
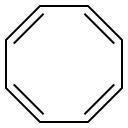
629-20-9
122 suppliers
$24.00/1g
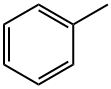
108-88-3
0 suppliers
$14.00/100ml

123-54-6
582 suppliers
$10.00/25ml
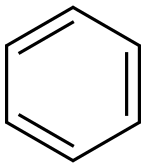
71-43-2
717 suppliers
$11.19/25ML