
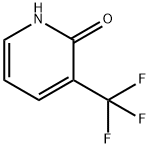
2-CHLORO-5-NITRO-3-(TRIFLUOROMETHYL)PYRIDINE synthesis
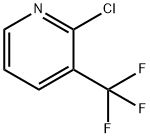
- Product Name:2-CHLORO-5-NITRO-3-(TRIFLUOROMETHYL)PYRIDINE
- CAS Number:99368-67-9
- Molecular formula:C6H2ClF3N2O2
- Molecular Weight:226.54
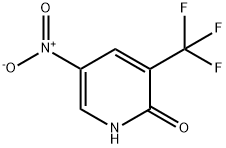
99368-66-8

99368-67-9
General procedure for the synthesis of 2-chloro-3-trifluoromethyl-5-nitropyridine from 2-hydroxy-5-nitro-3-trifluoromethylpyridine: Thionyl chloride (SOCl2, 18.45 mL, 253 mmol) was slowly added dropwise to a reaction vial containing 5-nitro-3-(trifluoromethyl)pyridin-2-ol (2.63 g, 12.64 mmol). Subsequently, N,N-dimethylformamide (DMF, 1.957 mL, 25.3 mmol) was added as a catalyst. The reaction mixture was stirred at 100°C for 10 hours. Upon completion of the reaction, the reaction solution was concentrated under reduced pressure to remove excess thionyl chloride. The concentrated residue was extracted by partitioning between ethyl acetate (EA) and saturated sodium bicarbonate (NaHCO3) solution. The organic phases were combined, washed with saturated brine, dried over anhydrous magnesium sulfate (MgSO4), filtered and concentrated under reduced pressure to afford the crude product 2-chloro-5-nitro-3-(trifluoromethyl)pyridine (2.46 g, 10.86 mmol, 86% yield), which could be used in the next reaction without further purification. Thin layer chromatography (TLC) analysis conditions: petroleum ether/ethyl acetate (PE/EA = 5:1), Rf = 0.6. 1H NMR (400 MHz, CDCl3) δ: 9.23-9.59 (m, 1H), 8.79 (d, J = 2.4 Hz, 1H).

99368-66-8
209 suppliers
$5.00/250mg

99368-67-9
137 suppliers
$7.00/250mg
Yield:99368-67-9 86%
Reaction Conditions:
with thionyl chloride in N,N-dimethyl-formamide at 100; for 10 h;Temperature;
Steps:
8.2 2-Chloro-5-nitro-3-(trifluoromethyl)pyridine
SOCl2 (18.45 mL, 253 mmol) was added to a solution of 5-nitro-3-(trifluoromethyl)pyridin-2-ol (2.63 g, 12.64 mmol). DMF (1.957 mL, 25.3 mmol) was added and the mixture was at 100° C. for 10 h. Then the solution was concentrated and distributed between EA and saturated NaHCO3 solution. The combined organic extract was washed with brine, dried over MgSO4, filtered and concentrated. The resulting 2-chloro-5-nitro-3-(trifluoromethyl)pyridine (2.46 g, 10.86 mmol, 86% yield) was used in the next step without further purification. TLC (PE/EA=5:1, Rf=0.6): 1H NMR (400 MHz, CDCl3) δ: 9.23-9.59 (m, 1H), 8.79 (d, J=2.4 Hz, 1H).
References:
GlaxoSmithKline Intellectual Property Development Limited;EIDAM, Hilary Schenck;Raha, Kaushik;Gong, Zhen;Guan, Huiping;Wu, Chengde;Yang, Haiying;Yu, Haiyu;Zhang, Zhiliu;CHEUNG, Mui US2014/275111, 2014, A1 Location in patent:Paragraph 0259; 0260
680-15-9
252 suppliers
$13.00/5g
25391-60-0
52 suppliers
$27.00/100mg

99368-67-9
137 suppliers
$7.00/250mg

99368-66-8
209 suppliers
$5.00/250mg

99368-67-9
137 suppliers
$7.00/250mg
22245-83-6
306 suppliers
$6.00/5g

99368-67-9
137 suppliers
$7.00/250mg
65753-47-1
377 suppliers
$8.00/5g

99368-67-9
137 suppliers
$7.00/250mg