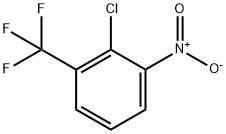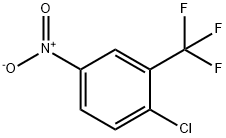
2-Chloro-5-nitrobenzotrifluoride synthesis
- Product Name:2-Chloro-5-nitrobenzotrifluoride
- CAS Number:777-37-7
- Molecular formula:C7H3ClF3NO2
- Molecular Weight:225.55
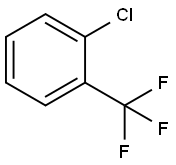
88-16-4

777-37-7
1) 400 g of o-chlorobenzotrifluoride (Feedstock I) was added to Preheating Module 2. Concentrated sulfuric acid (Feedstock II) and concentrated nitric acid (Feedstock III) were added to Preheating Module 1 for mixing and preheating, respectively. Subsequently, feedstocks I, II and III were introduced into the reaction module set for nitrification reaction. The flow rate of feedstock I was controlled to be 15 mL/min, the flow rate of feedstock II to be 18 mL/min, and the flow rate of feedstock III to be 11.5 mL/min by adjusting the flow pump. the reaction temperature was maintained at 60°C. The reaction temperature was maintained at 60°C. The flow rate of feedstock I was controlled to be 15 mL/min. The molar ratio of o-chlorobenzotrifluoride to nitric acid was 1:1.2, and the mass ratio of concentrated nitric acid to concentrated sulfuric acid was 1:4.0, and the reaction residence time was 55 seconds. The temperature of the cooling module was set to 25°C. The reaction solution at the outlet of the cooling module was collected for liquid-liquid separation. The product was combined with the organic phase by adding 270 mL of dichloromethane to the acid phase. It was washed with saturated sodium bicarbonate solution to neutrality and subsequently dried with anhydrous sodium sulfate. After evaporation of the solvent under reduced pressure, 480.36 g of 2-chloro-5-nitrobenzotrifluoride was obtained as a yellow oil. The yield was 96.13% and the purity was 99.29%.
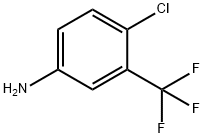
320-51-4
465 suppliers
$6.00/10g

777-37-7
293 suppliers
$10.00/5g
Yield:777-37-7 75%
Reaction Conditions:
with dihydrogen peroxide;potassium carbonate in water;acetonitrile at 20;
Steps:
I General procedure for the synthesis of substituted nitrobenzenes (2a-z).
General procedure: To a solution of p-toluidine (1.0 mmol) and potassium carbonate (1.0 mmol) in CH3CN (3 mL) were added a solution of 50% aqueous H2O2 (3.0 mmol) and the mixture was stirred at room temerature for 10-15 min. The mixture was then dried to vacuum and extracted three times with ethyl acetate followed by washing with brine,and dried over anhydrous Na2SO4. Evaporation of the solvent under vacuum afforded the crude product, which was furthur purified by column chromatography using hexane/ethyl acetate mixture and then analyzed by spectroscopy.
References:
Gupta, Sampa;Ansari, Alisha;Sashidhara, Koneni V. [Tetrahedron Letters,2019,vol. 60,# 39,art. no. 151076] Location in patent:supporting information

88-16-4
267 suppliers
$13.00/25g

777-37-7
293 suppliers
$10.00/5g