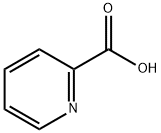
2-Picolinic acid synthesis
- Product Name:2-Picolinic acid
- CAS Number:98-98-6
- Molecular formula:C6H5NO2
- Molecular Weight:123.11
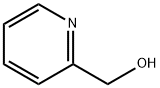
586-98-1
416 suppliers
$6.00/10g

98-98-6
627 suppliers
$5.00/10g
Yield:98-98-6 97%
Reaction Conditions:
with tert-butyldimethylsilyl chloride in methanol;water at 20; for 0.5 h;Green chemistry;
Steps:
Synthesis of carboxylic acids pyridine-2-carboxylic acid (7), thiophene-2-carboxylic acid (8) or furan-2-carboxylic acid (9)
An appropriate alcohol [2-pyridinemethanol (3) (1.0 g, 9.20 mmol, 1 eq), 2-thiophenemethanol (4) (1.0 g, 8.78 mmol, 1 eq) or 2-furanmethanol (5) (1.0 g,10.20 mmol] was added to reaction mixture that contained Ag/ZnO/graphene nanocomposite (10 mg, 1 wt%), and TBSCl (1.38 g, 9.20 mmol, 1 eq), (1.32 g, 8.78 mmol, 1 eq), (1.53 g, 10.20 mmol, 1 eq) or (1.38 g, 9.26 mmol, 1 eq) dissolved in MeOH:H2O (1:1, 10 mL). The mixture was stirred for 30, 60 or 60 min, respectively, at room temperature and monitored by TLC using eluent system (EtOAc:Hexane, 3:7). The mixture was filtrated and then water (5 mL) was added, and the mixture was extracted with (CHCl3: MeOH, 1:1) (5 mL * 2). The collected methanol/water layer was evaporated using rotary evaporator to afford the residue that was purified by column chromatography using silica gel (Hexane:EtOAc, 7:3, 6:4 and then 1:1) to afford the corresponding carboxylic acids; pyridine-2-carboxylic acid (7); thiophene-2-carboxylic acid (8) or furan-2-carboxylicacid (9) respectively.
References:
Attia, Yasser A.;Vázquez, Carlos Vázquez;Mohamed, Yasser M. A. [Research on Chemical Intermediates,2017,vol. 43,# 1,p. 203 - 218]
100-70-9
426 suppliers
$13.00/25g

98-98-6
627 suppliers
$5.00/10g
![2-Pyridinemethanol, α-[2-(4-methoxyphenyl)cyclopropyl]-](/CAS/20210305/GIF/1309453-54-0.gif)
1309453-54-0
0 suppliers
inquiry

98-98-6
627 suppliers
$5.00/10g
METHANONE](/StructureFile/ChemBookStructure4/GIF/CB9323704.gif)
338415-92-2
11 suppliers
inquiry
100-09-4
662 suppliers
$6.00/25g
109-06-8
414 suppliers
$16.00/25g

98-98-6
627 suppliers
$5.00/10g
1121-60-4
515 suppliers
$10.60/10gm:

98-98-6
627 suppliers
$5.00/10g