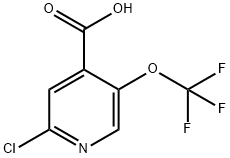
2-chloro-5-(trifluoroMethoxy)isonicotinic acid synthesis
- Product Name:2-chloro-5-(trifluoroMethoxy)isonicotinic acid
- CAS Number:1221171-77-2
- Molecular formula:C7H3ClF3NO3
- Molecular Weight:241.55

124-38-9
134 suppliers
$214.00/14L
1206972-45-3
78 suppliers
$59.00/250mg

1221171-77-2
22 suppliers
$200.00/25mg
Yield:1221171-77-2 75%
Reaction Conditions:
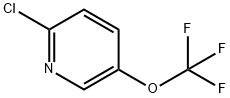
Stage #1: 2-chloro-5-(trifluoromethoxy)pyridinewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78;
Stage #2: carbon dioxide in tetrahydrofuran;hexane at 25;
Stage #3: with hydrogenchloride in water; pH=4;
Steps:
1
1. Functionalized 3-OCF3-pyridine:; 2-Chloro-5-trifluoromethoxy isonicotinic acid (17); At 0 0C, diisopropylamine (0.25 g, 0.35 mL, 2.5 mmol, 1 eq) was added dropwise to a solution of butyllithium (1.56 M in hexane, 1.6 mL, 2.5 mmol, 1 eq) in THF (4 mL). At -78 0C, a solution of 2-chloro-5-trifluoromethoxypyridine (6, 0.5 g, 2.5 mmol, 1 eq) in THF (2 mL) was added dropwise and the reaction mixture was stirred for 2 h at this temperature. Then, the mixture was poured onto an excess of freshly crushed dry ice and allowed to reach 25 0C before being treated with an aqueous solution of sodium hydroxide (5%, 5 mL). The resulting aqueous layer was collected, washed with diethylether (2 mL) and acidified to pH 4 by dropwise addition of hydrochloric acid (6 N, 1 mL) before being extracted with ethyl acetate (3 >< 3 mL). The combined organic layers were dried over sodium sulfate and the solvents evaporated to afford pure 2- chloro-5-trifluoromethoxy isonicotinic acid (17, 0.46 g, 1.9 mmol, 75%) as a white powder; m.p. 150-152 0C.1H NMR (CDCl3, 300 MHz): δ = 8.45 (s, 1 H), 7.84 (s, 1 H), 3.88 (br s, IH). - 19F NMR (CDCl3, 282 MHz): δ = -57.8. - 13C NMR (CDCl3, 75 MHz): δ = 150.3, 144.9, 142.5, 137.1, 133.8, 126.1, 120.3 (q, J = 260 Hz). - MS(APCI+): m/z = 241 [M+], 197 [M+-CO2].
References:
WO2010/40461,2010,A1 Location in patent:Page/Page column 31-32