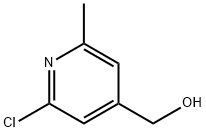
(2-chloro-6-methylpyridin-4-yl)methanol synthesis
- Product Name:(2-chloro-6-methylpyridin-4-yl)methanol
- CAS Number:152815-18-4
- Molecular formula:C7H8ClNO
- Molecular Weight:157.6
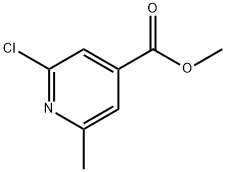
3998-90-1
200 suppliers
$4.00/1g

152815-18-4
20 suppliers
inquiry
Yield:152815-18-4 93%
Reaction Conditions:
Stage #1: 2-chloro-6-methyl-isonicotinic acid methyl esterwith lithium aluminium tetrahydride in tetrahydrofuran at 20; for 2.4 h;
Stage #2: with water in tetrahydrofuran;
Steps:
1.1
LiAlH4 (10.0 g, 0.264 mol, 1.24 eq) was added portionwise to a solution of methyl-2-chloro-6-methylpyridine-4-carboxylate 1 (39.62 g, 0.213 mol) in dry THF (800 mL) at room temperature with stirring over a period of 1.4 h. The resulting mixture was stirred for 1 h and quenched with water. 15% aqueous NaOH (100 mL) was added, followed by aqueous sodium-potassium tartrate (1 L). The resulting mixture was stirred for a further 1.25 h and extracted with dichloromethane (2×1 L) to give, after concentration, (2-chloro-6-methylpyridin-4-yl)-methanol 2 (31.06 g, 93%) as a yellow solid.
References:
US2007/66644,2007,A1 Location in patent:Page/Page column 8
25462-85-5
234 suppliers
$6.00/250mg

152815-18-4
20 suppliers
inquiry
3998-88-7
87 suppliers
$41.20/250mg

152815-18-4
20 suppliers
inquiry