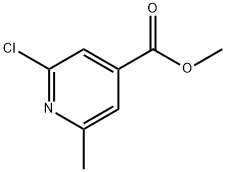
Methyl 2-chloro-6-methylpyridine-4-carboxylate synthesis
- Product Name:Methyl 2-chloro-6-methylpyridine-4-carboxylate
- CAS Number:3998-90-1
- Molecular formula:C8H8ClNO2
- Molecular Weight:185.61

67-56-1
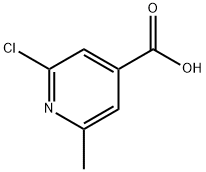
25462-85-5

3998-90-1
General procedure: 2-chloro-6-methylpyridine-4-carboxylic acid (2.37 g, 15.5 mmol) was added to a stirred solution of phosphorus oxychloride (POCl3, 10 mL, 107 mmol) and heated to reflux for 18 hours. Upon completion of the reaction, excess POCl3 was removed by distillation under reduced pressure and the residue was cooled to -5°C. Methanol (20 mL) was slowly added dropwise and stirred for 24 h at room temperature. Subsequently, methanol was removed by complete distillation and the reaction mixture was neutralized with solid sodium bicarbonate (NaHCO3) and partitioned between water (30 mL) and ethyl acetate (EtOAc, 30 mL). The aqueous layer was extracted with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure. The crude product was purified by rapid chromatography on silica gel (eluent: dichloromethane, CH2Cl2) to afford methyl 2-chloro-6-methyl-isonicotinate (2 g, 10.8 mmol, 69% yield) as a white solid. Thin layer chromatography (TLC, silica gel plate, dichloromethane as unfolding agent) Rf value was 0.66. Nuclear magnetic resonance hydrogen spectrum (1H NMR, 300 MHz, CDCl3) δ 2.59 (s, 3H), 3.94 (s, 3H), 7.61 (s, 1H), 7.67 (s, 1H); nuclear magnetic resonance carbon spectrum (13C NMR, 75 MHz, CDCl3) δ 24.2, 52.9, 120.8, 121.2, 140.3, 151.4, 160.5, 164.6; mass spectrum (MS, EI, 70 eV): m/z (relative abundance) [M+H]+ 186 (100).

25462-85-5
231 suppliers
$6.00/250mg

79-37-8
490 suppliers
$17.67/10gm:

3998-90-1
202 suppliers
$4.00/1g
Yield:3998-90-1 100%
Reaction Conditions:
in methanol;dichloromethane
Steps:
63 Example 63
The starting material was prepared as follows: Oxalyl chloride (1.9 g, 15 mmol) was added to 2-chloro-6-methyl-pyridine4-carboxylic acid (1.7 g, 10 mmol) in methylene chloride (30 ml) and the mixture stirred for 2 hours. The volatiles were removed by evaporation and methanol (20 ml) added to the residue. The mixture was stirred for 1 hour and the volatiles removed by evaporation to give methyl 2-chloro-6-methyl-pyridine-4-carboxylate (1.85 g, 100%) as an off-white solid. 1 H NMR Spectrum: (CDCl3) 2.55(s, 3H); 3.90(s, 3H); 7.55(s, 1H); 7.60(s, 1H); MS - ESI: 186 [MH]+
References:
Zeneca Limited;Zeneca Pharma S.A., US5962458, 1999, A

67-56-1
790 suppliers
$7.29/5ml-f
86454-13-9
104 suppliers
$10.00/250mg

3998-90-1
202 suppliers
$4.00/1g

67-56-1
790 suppliers
$7.29/5ml-f

25462-85-5
231 suppliers
$6.00/250mg

3998-90-1
202 suppliers
$4.00/1g
98491-78-2
55 suppliers
$24.00/250 mg

3998-90-1
202 suppliers
$4.00/1g

86454-13-9
104 suppliers
$10.00/250mg

3998-90-1
202 suppliers
$4.00/1g