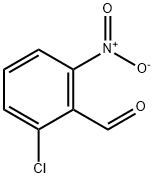
2-CHLORO-6-NITROBENZALDEHYDE synthesis
- Product Name:2-CHLORO-6-NITROBENZALDEHYDE
- CAS Number:6361-22-4
- Molecular formula:C7H4ClNO3
- Molecular Weight:185.56
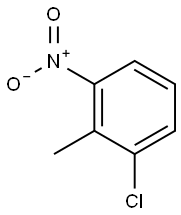
83-42-1
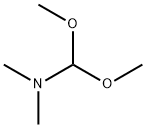
4637-24-5

6361-22-4
This embodiment relates to the synthesis of 2-chloro-6-nitrobenzaldehyde: To a stirred solution of 2-chloro-6-nitrotoluene (18.53 g, 108.0 mmol) in anhydrous N,N-dimethylformamide (240 mL) was added N,N-dimethylformamide dimethyl acetal (39.5 g, 44.0 mL, 331.0 mmol). After the mixture was heated at 140 °C for 16 h, the dark red solution was cooled to 0 °C and slowly added to a rapidly stirred solution of sodium periodate (83.0 g, 388.0 mmol) in water (291 mL) through a cannula over a 1 h period. The reaction flask was washed with N,N-dimethylformamide (77 mL) at 0 °C and the washings were added to the sodium periodate mixture. The reaction mixture was stirred at 0°C for 2 hours and then slowly warmed to room temperature. After continued stirring for 6 hours, the orange solution was filtered and rinsed with toluene/ethyl acetate (1:1, 200 mL). The filtrate was washed sequentially with water (3 x 150 mL) and saturated aqueous sodium chloride solution (3 x 150 mL). The organic phase was dried with magnesium sulfate and concentrated under reduced pressure to give a dark red oil, which precipitated as a solid upon addition of hexane (40 mL). The solid was recrystallized by toluene to afford the target product 2-chloro-6-nitrobenzaldehyde (17.23 g, 92.88 mmol, 86% yield).1H NMR (400 MHz, CDCl3) δ 10.42 (s, 1H), 8.01 (dd, J=1.0, 8.2 Hz, 1H), 7.79 (dd, J=1.0, 8.1 Hz, 1H ), 7.65 (t, J=8.1Hz, 1H); 13C NMR (100MHz, CDCl3) δ 188.6, 148.4, 138.6, 132.9, 132.4, 123.4, 121.9.

83-42-1
278 suppliers
$9.00/5g

4637-24-5
646 suppliers
$5.00/25g

6361-22-4
137 suppliers
$17.00/250mg
Yield: 86%
Reaction Conditions:
Stage #1:6-chloro-2-nitrotoluene;N,N-dimethyl-formamide dimethyl acetal in N,N-dimethyl-formamide at 140; for 16 h;
Stage #2: with sodium periodate in water;N,N-dimethyl-formamide at 0 - 20; for 9 h;
Steps:
109
Example 109; This example concerns the synthesis of Aldehyde 4: To a stirred solution of1 (18.53 g, 108.0 mmol) in dry DMF (240 mL) was added N,JV-dimethylformamide dimethyl acetal (DMF'DMA) (39.5 g, 44.0 mL, 331 mmol). After heating at 140°C for 16 h, the dark red solution was cooled to 0°C and added slowly, over 1 h via cannula, to a rapidly stirred solution OfNaIO4 (83.0 g, 388.0 mmol) in H2O (291 mL) and DMF (77 mL) at 0°C. The reaction flask was washed with DMF (20 mL) at 0°C and added to NaIO4 mixture. The reaction was stirred at 0°C for 2 h then allowed to warm to rt. After an additional 6 h, the orange solution was filtered and rinsed with PhMe/EtOAc (1 : 1 , 200 mL). The filtrate was then washed with H2O (3 x 150 mL) and sat. aq. NaCl (3 x 150 mL). The dried (MgSO4) extract was concentrated in vacuo to a dark red oil, and hexanes (40 mL) were added. Solids were isolated and recrystalized in PhMe to give the known aldehyde 4 (17.23 g, 92.88 mmol, 86%). 1H NMR (400 MHz, CDCl3) δ 10.42 (s, IH), 8.01 (dd, J= 1.0, 8.2 Hz, IH), 7.79 (dd, J= 1.0, 8.1 Hz5 IH), 7.65 (t, J= 8.1 Hz, IH); 13C NMR (100 MHz, CDCl3) δ 188.6, 148.4, 138.6, 132.9, 132.4, 123.4, 121.9.
References:
STATE OF OREGON ACTING BY AND THROUGH THE STATE BOARD OF HIGHER EDUCATION ON BEHALF OF OREGON STATE UNIVERSITY WO2008/156656, 2008, A2 Location in patent:Page/Page column 46; 177

83-42-1
278 suppliers
$9.00/5g

6361-22-4
137 suppliers
$17.00/250mg
50907-57-8
79 suppliers
$23.00/250mg

6361-22-4
137 suppliers
$17.00/250mg
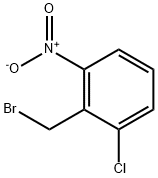
56433-01-3
74 suppliers
inquiry

6361-22-4
137 suppliers
$17.00/250mg
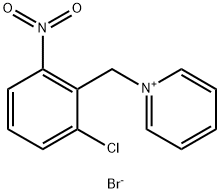
95196-89-7
3 suppliers
$53.00/250mg

6361-22-4
137 suppliers
$17.00/250mg