
2-chloro-6-(trichloroMethoxy)pyridine synthesis
- Product Name:2-chloro-6-(trichloroMethoxy)pyridine
- CAS Number:1221171-69-2
- Molecular formula:C6H3Cl4NO
- Molecular Weight:246.91
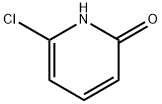
16879-02-0
180 suppliers
$11.00/5g
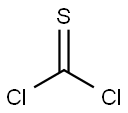
463-71-8
154 suppliers
$32.00/10g

1221171-69-2
12 suppliers
inquiry
Yield:1221171-69-2 63%
Reaction Conditions:
Stage #1: 6-chloro-2-hydroxypyridine;thiophosgenewith sodium hydroxide in chloroform at 0; for 2.5 h;
Stage #2: with chlorine in chloroform at 20; for 26 h;
Steps:
6.a; 7.a
Examples 6 and 7: Preparation of 3-hydroxy-4-(4-isopropylbenzoyl)-l-(6- methylpyridazin-3-yl)-5-(6-(trifluoromethoxy)pyridin-3-yl)-lH-pyrrol-2(5H)-one (Example 6) and its chlorhydrate (Example 7) a) 2-chloro-6-(trichloromethoxy)pyridine (1-4); 1 ) Thiophosgene, NaOH 5%,CHCk 0 °C. 2hΟΓ N OH 2) CI2, 24h ΟΓ N "OCCIA suspension of 6-chloropyridin-2-ol (15.3 g; 1 18 mmol; 1 eq) in NaOH 5% (75 mL; 130 mmol; 1.1 eq) and chloroform (53 mL), was cooled down at 0°C, thiophosgene (9.05 mL; 118 mmol; 1 eq) was added dropwise over half an hour and stirred at 0 °C for 2 hours. Additional chloroform (3 x 40 mL) was added and the combined organic layers were washed with HC1 IN (40 mL) and water (40 mL), dried over sodium sulphate and cautiously filtered. At room temperature, the filtrate was saturated with chlorine until the reaction mixture begins to warm up. After 2 hours at room temperature, another excess of chlorine was added until a yellow solution is obtained. After 24 hours at the same temperature, the excess of chlorine was removed by a stream of argon and the solvent was evaporated. The pale yellow oil obtained was distilled under vacuum (P = 4 mbar; T° = 141°C) to afford 2-chloro-6-(trichloromethoxy)pyridine in 63 % yield as a pale yellow oil.1H-NMR (CDC13): δ (ppm) 7.10 (d, J = 8.4 Hz, 1H); 7.29 (d, J = 7.6 Hz, 1H); 7.77 (t, J = 8Hz, 1H).
References:
WO2012/93174,2012,A1 Location in patent:Page/Page column 37

16879-02-0
180 suppliers
$11.00/5g

463-71-8
154 suppliers
$32.00/10g

1221171-69-2
12 suppliers
inquiry