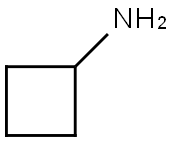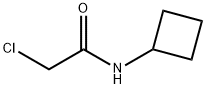
2-chloro-N-cyclobutylacetamide synthesis
- Product Name:2-chloro-N-cyclobutylacetamide
- CAS Number:1192687-51-6
- Molecular formula:C6H10ClNO
- Molecular Weight:147.6
Yield:1192687-51-6 83%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 10; for 1 h;
Steps:
4.1.6 General procedure for synthesis of chloroacetamides (70a-h)
General procedure: To the appropriate substituted amines (83a-h, 1 equiv) in DCM was added chloroacetyl chloride 84 (1.1 equiv) followed by the addition of TEA (2.8 equiv) at 0-10°C. The reaction was continued at the same temperature for 1h. Then, the reaction mixture was washed with saturated NaHCO3 solution, HCl 2N, and brine solution. The excess of organic solvent was removed under reduced pressure and the crude compounds were purified by flash chromatography, eluting with Cyclohexane/EtOAc 1/1. 2-chloro-N-cyclobutylacetamide (70a). Cyclobutylamine 83a (240μL, 2.81mmol), chloroacetyl chloride 84 (248μL, 3.12mmol), TEA (1.09mL, 7.8mmol) in DCM (6mL) were allowed to react according to the general procedure. Clean compound was eluted with Cycloexane/EtOAc 7/3, giving 70a (380mg, 83% yield) as white solid. 1H NMR (400MHz, DMSO-d6) δ 8.43 (s, 1H), 4.28-4.09 (m, 1H), 3.98 (s, 2H), 2.21-2.08 (m, 2H), 2.00-1.80 (m, 2H), 1.70-1.59 (m, 2H) ppm. UPLC-MS (ESI, m/z) Rt=1.23min-148 [M (35Cl)+H]+.
References:
Roberti, Marinella;Schipani, Fabrizio;Bagnolini, Greta;Milano, Domenico;Giacomini, Elisa;Falchi, Federico;Balboni, Andrea;Manerba, Marcella;Farabegoli, Fulvia;De Franco, Francesca;Robertson, Janet;Minucci, Saverio;Pallavicini, Isabella;Di Stefano, Giuseppina;Girotto, Stefania;Pellicciari, Roberto;Cavalli, Andrea [European Journal of Medicinal Chemistry,2019,vol. 165,p. 80 - 92]