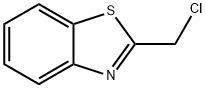
2-(CHLOROMETHYL)-1,3-BENZOTHIAZOLE synthesis
- Product Name:2-(CHLOROMETHYL)-1,3-BENZOTHIAZOLE
- CAS Number:37859-43-1
- Molecular formula:C8H6ClNS
- Molecular Weight:183.66
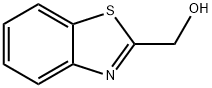
37859-42-0

37859-43-1
General procedure for the synthesis of 2-(chloromethyl)-1,3-benzothiazole from 2-hydroxymethylbenzothiazole: To a stirred solution containing 2-hydroxymethylbenzothiazole (2.5 g, 15.1 mmol), triphenylphosphine (4.89 g, 18.6 mmol), and anhydrous toluene (50 ml) was added, protected by nitrogen, carbon tetrachloride (15.0 ml, 155 mmol ). The reaction mixture was heated to reflux for 5 hours. After completion of the reaction, the reaction mixture was concentrated using a rotary evaporator to give the crude product. The crude product was purified by rapid chromatography on silica gel to afford 2-(chloromethyl)-1,3-benzothiazole (2.36 g, 85% yield) using 100% dichloromethane as eluent. The product was confirmed by mass spectrometry (ion spray): m/z = 183.9 (M + 1); 1H NMR (CDCl3) data were as follows: δ= 8.04-8.01 (1H, m), 7.91-7.89 (1H, m), 7.53-7.49 (1H, m), 7.44-7.40 (1H, m), 4.95 (2H, s).

79-04-9
384 suppliers
$12.00/5g
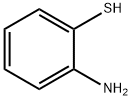
137-07-5
314 suppliers
$10.00/5g

37859-43-1
117 suppliers
$12.00/250mg
Yield: 86.8%
Reaction Conditions:
for 0.166667 h;Microwave irradiation;
Steps:
Synthesis of 2-Chloromethyl-benzothiazole (4) [27].
To a solution of 2-aminobenzenethiol (1 g,7.93 mmol) in acetic acid (15 mL), and 2-chloroacetyl chloride (1.35 g, 1.19 mmol) was added dropwise.The reaction mixture was irradiated in a microwave oven (Ethos start) for 10 min at a power of 500 W.After cooling, the mixture was poured onto crushed ice (100 mL) and basified with 5 mol/L NaOH.The solution was extracted with chloroform (3 50 mL). The combined organic layers were dried overMgSO4 and concentrated under vacuum. Purification of the residue by column chromatography onsilica gel (petroleum ether/acetone, 10:1 v:v) gave compound 4 as a yellow solid (1.90 g, 86.8%) [27].m.p., 89-90 °C; MS (+ESI) m/z 184.02 [M + H]+.
References:
Luo, Bo;Li, Ding;Zhang, An-Ling;Gao, Jin-Ming [Molecules,2018,vol. 23,# 10,art. no. 2457]

37859-42-0
123 suppliers
$25.00/250mg

37859-43-1
117 suppliers
$12.00/250mg
120-75-2
237 suppliers
$10.00/5g

37859-43-1
117 suppliers
$12.00/250mg
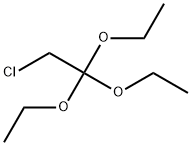
51076-95-0
133 suppliers
$43.50/1g

137-07-5
314 suppliers
$10.00/5g

37859-43-1
117 suppliers
$12.00/250mg
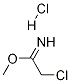
70737-12-1
26 suppliers
$83.09/5g

137-07-5
314 suppliers
$10.00/5g

37859-43-1
117 suppliers
$12.00/250mg