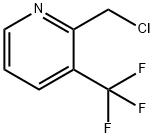
2-(CHLOROMETHYL)-3-(TRIFLUOROMETHYL)PYRIDINE synthesis
- Product Name:2-(CHLOROMETHYL)-3-(TRIFLUOROMETHYL)PYRIDINE
- CAS Number:215867-86-0
- Molecular formula:C7H5ClF3N
- Molecular Weight:195.57
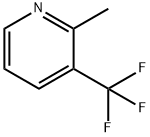
106877-18-3
19 suppliers
$39.00/250mg

215867-86-0
34 suppliers
inquiry
Yield:215867-86-0 75%
Reaction Conditions:
with 1-hydroxy-pyrrolidine-2,5-dione;N-chlorophthalimide;2,2'-azobis(isobutyronitrile) in 1,2-dichloro-ethane at 20 - 80; for 4 h;Temperature;Reagent/catalyst;Solvent;
Steps:
1-3
N-chloroisoindole-1,3-dione 10mmol (1.33g),Azobisisobutyronitrile (AIBN) 0.1mmol (16mg),30mmol (4.83g) of 2-methyl-3-trifluoromethylpyridine and 1mmol (163mg) of N-hydroxysuccinimide were dissolved in 40mL of dichloroethane and stirred at room temperature for 5min. Then the temperature was raised to 80°C and the reaction was continued for 4h. After the reaction, the acetonitrile was removed by rotary evaporation, and purified by silica gel column chromatography to obtain the final 2-chloromethyl-3-fluoromethylpyridine, 4.40 g, with a yield of 75% and a purity of 97.0%.
References:
CN112279802,2021,A Location in patent:Paragraph 0023-0028
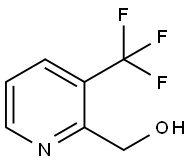
131747-44-9
62 suppliers
$350.00/250mg

215867-86-0
34 suppliers
inquiry