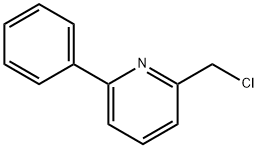
2-(CHLOROMETHYL)-6-PHENYLPYRIDINE synthesis
- Product Name:2-(CHLOROMETHYL)-6-PHENYLPYRIDINE
- CAS Number:147937-33-5
- Molecular formula:C12H10ClN
- Molecular Weight:203.67
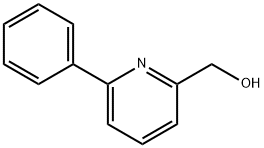
162614-73-5
5 suppliers
inquiry

147937-33-5
1 suppliers
inquiry
Yield:147937-33-5 99%
Reaction Conditions:
with thionyl chloride at 20; for 1 h;
Steps:
108 Reference Example 108
A mixture of 6-phenyl-2-pyridylmethanol (1.76 g) and thionyl chloride (10 ml) was stirred at room temperate for 1 hour. The reaction mixture was concentrated under reduced pressure, and aqueous saturated sodium bicarbonate solution was added to the residue, which was extracted with ethyl acetate. The ethyl acetate layer was washed with saturated aqueous sodium chloride solution, dried (MgSO4), and concentrated. The residue was subjected to silica gel column chromatography to obtain 2-chloromethyl-5-phenylpyridine (1.91 g, yield 99%) as a colorless oily substance from the fraction eluted with ethyl acetate-hexane (1:5, volume ratio). NMR(CDCl3)δ : 4.75(2H, s), 7.36-7.52(4H, m), 7.64(1H, dd, J=1.0, 7.6 Hz), 7.77(1H, t, J=7.6 Hz), 7.96-8.02(2H, m).
References:
EP1228067,2004,B1 Location in patent:Page 60
5315-25-3
468 suppliers
$6.00/5g

147937-33-5
1 suppliers
inquiry
46181-30-0
106 suppliers
$28.60/25MG

147937-33-5
1 suppliers
inquiry
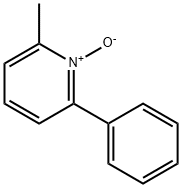
20531-89-9
0 suppliers
inquiry

147937-33-5
1 suppliers
inquiry

98-80-6
746 suppliers
$5.00/5g

147937-33-5
1 suppliers
inquiry