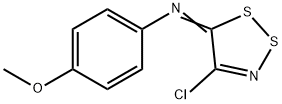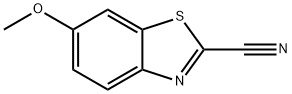
2-Cyano-6-methoxybenzothiazole synthesis
- Product Name:2-Cyano-6-methoxybenzothiazole
- CAS Number:943-03-3
- Molecular formula:C9H6N2OS
- Molecular Weight:190.22
32338-02-6
138 suppliers
$14.00/1g
75318-43-3
61 suppliers
$32.00/100mg

943-03-3
214 suppliers
$9.00/100mg
Yield:943-03-3 64%
Reaction Conditions:
Stage #1:2-bromo-4-methoxyaniline;4,5-dichloro-1,2,3-dithiazolium chloride in dichloromethane at 20; for 1 h;Inert atmosphere;
Stage #2: with 1,8-diazabicyclo[5.4.0]undec-7-ene in dichloromethane at 5 - 40; for 5 h;Inert atmosphere;
Steps:
General procedure for the one-pot, base-mediated, metal free synthesis of 6-substituted 2-c yanobenzothiazoles from monobrominated para -substituted anhlines
General procedure: Appel’s salt and an aniline were allowed to stir in CH2CI2 (DCM) for 1 h at room temperature under a nitrogen atmosphere. The solution was then cooled to below 5 00 and base (preferably DBU orDBN) was added, dropwise over 30 mm, to the stirring solution maintained at 5 00 all under a nitrogen atmosphere. After the addition, the resulting mixture was stirred for 30 mm while allowing it to warm to room temperature, after which it was ref luxed at 40 C for 4 h. Upon cooling to room temperature (rt), ethyl acetate (EtOAc) was added. The reaction mixture was then washed with saturated NH4CIaq solution, and H20. The organic phase was dried over Na2SO4 and concentratedin vacuo. The crude material was purified by silica gel chromatography to provide the corresponding benzothiazoles.
References:
UNIVERSITY OF CAPE TOWN;JARDINE, Moegamat Anwar;RYLANDS, Marwaan WO2019/21202, 2019, A1 Location in patent:Page/Page column 26
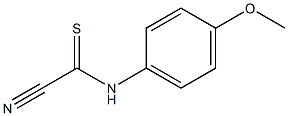
4968-41-6
0 suppliers
inquiry

943-03-3
214 suppliers
$9.00/100mg
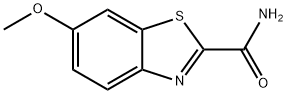
946-12-3
45 suppliers
$168.00/1g

943-03-3
214 suppliers
$9.00/100mg
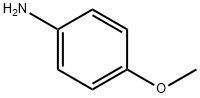
104-94-9
497 suppliers
$9.00/1g

75318-43-3
61 suppliers
$32.00/100mg
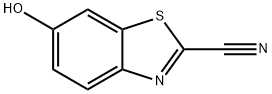
939-69-5
220 suppliers
$36.00/1g

943-03-3
214 suppliers
$9.00/100mg