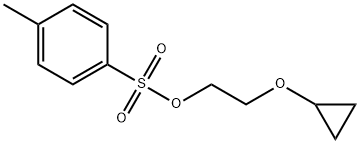
2-Cyclopropoxyethyl4-methylbenzenesulfonate synthesis
- Product Name:2-Cyclopropoxyethyl4-methylbenzenesulfonate
- CAS Number:862728-59-4
- Molecular formula:C12H16O4S
- Molecular Weight:256.32
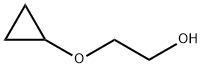
20117-44-6
22 suppliers
inquiry

98-59-9
624 suppliers
$9.00/5g

862728-59-4
63 suppliers
$41.00/250mg
Yield:862728-59-4 76%
Reaction Conditions:
Stage #1: 2-(cyclopropyloxy)ethan-1-olwith sodium hydroxide in tetrahydrofuran;2-methyltetrahydrofuran;water at -5 - 0; for 1 h;Inert atmosphere;
Stage #2: p-toluenesulfonyl chloride in tetrahydrofuran;water at 0 - 5; for 5 h;Inert atmosphere;Solvent;Temperature;
Steps:
7 Preparation of 2-cyclopropoxyethyl 4-methylbenzenesulfonate (2)
Preparation of 2-cyclopropoxyethyl 4-methylbenzenesulfonate (2) [0183] 2-cyclopropoxyethanol in THF and 2-methyltetrahydrofuran. The mixture was cooled to -5° C. to 0° C. with stirring (100 rpm). A precooled (0° C.) aqueous solution of NaOH (134 g, 3.3 mole) in water (580 mL) was added dropwise to the mixture over 40 minutes while keeping the internal temperature between -5° C. and 0° C. The mixture was stirred for another 20 minutes at -5° C. to 0° C. 4-Methyl-benzenesulfonyl chloride (252 g, 1.32 mole) was added portion-wise over 40 minutes while keeping the internal temperature between -5° C. and 0° C. The reaction was stirred at -5° C. to 0° C. under nitrogen for another 5 hours in a cooling bath. The cooling bath was removed and the reaction was warmed slowly to 10° C. to 20° C. and stirred overnight. [0185] Brine (400 mL) was added to the reaction. The mixture was extracted with ethyl acetate/petroleum ether (fractions: 60° C. to 90° C.) (5:1 v/v, 300 mL×3). The combined organic layers were washed with saturated brine (300 mL) and water (200 mL), and then concentrated under reduced pressure at 40° C. to give the crude product as a liquid (215.6 g, yield: 76%, purity: 99.0%. To the crude product was added 200 g of ethanol and evaporated under reduced pressure (0.1 MPa) at 40° C. to remove any residual solvents.
References:
US2015/210634,2015,A1 Location in patent:Paragraph 0183-0185
18742-02-4
303 suppliers
$6.00/5g

862728-59-4
63 suppliers
$41.00/250mg