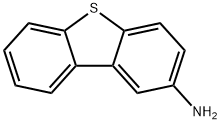
2-Dibenzothiophenamine synthesis
- Product Name:2-Dibenzothiophenamine
- CAS Number:7428-91-3
- Molecular formula:C12H9NS
- Molecular Weight:199.27
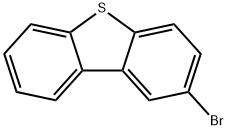
22439-61-8
243 suppliers
$6.00/1g

7428-91-3
54 suppliers
$78.00/200MG
Yield:7428-91-3 68%
Reaction Conditions:
Stage #1: 2-bromodibenzothiophenewith Benzophenone imine;2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl;bis(dibenzylideneacetone)-palladium(0);sodium t-butanolate in toluene; for 12 h;Reflux;
Stage #2: with hydrogenchloride in water;
Steps:
2 Intermediate Synthesis Example 2: Synthesis of Intermediate (4)
2-bromodibenzo[b,d]thiophene (2-bromodibenzo[b,d]thiophene) 50.0 g (190.0 mmol), benzophenone imine 37.8 g (208.6 mmol), Pd (dba) 25.5 g (9.5 mmol), 11.8 g (19.0 mmol) of BINAP, 45.6 g (474.5 mmol) of sodium tert-butoxy and 800 mL of toluene were stirred under reflux for 12 hours. The reaction mixture was cooled to room temperature and dissolved in chloroform. The solution was passed through a celite pad and concentrated under reduced pressure. After the obtained compound was suspended in 600 mL of tetrahydrofuran, 30 mL of concentrated hydrochloric acid was slowly added thereto, followed by stirring at room temperature overnight. The resulting precipitate was filtered under reduced pressure and washed with chloroform. The filtered wet body was suspended in 300 mL of water, the pH was adjusted to 8 or higher with a saturated sodium carbonate solution, and the layers were separated by extraction with chloroform. The separated chloroform layer was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The concentrated residue was slurried with dichloromethane and n-hexane to obtain 25.6 g (yield: 68.0%) of the compound (intermediate (4)) as a yellow solid.
References:
KR2022/30351,2022,A Location in patent:Paragraph 0241-0243
6639-36-7
40 suppliers
$70.00/100mg

7428-91-3
54 suppliers
$78.00/200MG
22439-58-3
4 suppliers
inquiry

7428-91-3
54 suppliers
$78.00/200MG
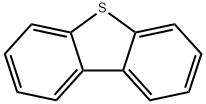
132-65-0
368 suppliers
$6.00/5g

7428-91-3
54 suppliers
$78.00/200MG

6639-36-7
40 suppliers
$70.00/100mg

7428-91-3
54 suppliers
$78.00/200MG
2243-47-2
216 suppliers
$6.00/1g