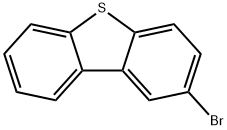
2-BROMODIBENZOTHIOPHENE synthesis
- Product Name:2-BROMODIBENZOTHIOPHENE
- CAS Number:22439-61-8
- Molecular formula:C12H7BrS
- Molecular Weight:263.15
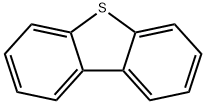
132-65-0

22439-61-8
9.2 g of dibenzothiophene was placed in a 500 mL three-necked round-bottomed flask and 150 mL of dichloroethane was added. The dibenzothiophene was dissolved under magnetic stirring and the temperature of the reaction system was adjusted to 10 °C. To the reaction system, 10.7 g of N-bromosuccinimide and 3 g of acidic clay (pH 3, measured as a 10% aqueous solution) were sequentially added for 6 hours. The amount of acidic clay was 32.6 wt% of the mass of dibenzothiophene. Upon completion of the reaction, 150 mL of 5% aqueous sodium bicarbonate was added to the reaction mixture, stirred for 1 h and left to stratify. The organic phase was washed twice with water, followed by spinning to remove all solvents to give the crude product as a solid.HPLC analysis (254 nm wavelength) showed 96.71 wt% of 2-bromodibenzothiophene in the crude product, 2.21 wt% of the by-product 2,7-dibromodibenzothiophene, and 0.01 wt% of unreacted dibenzothiophene. The crude product was dissolved in 300 mL of acetonitrile and heated to 82 °C to dissolve it completely. After slow cooling to 30 °C, the precipitated 2,7-dibromodibenzothiophene by-product was removed by filtration. The filtrate was vortexed to 310 mL and cooled to room temperature to precipitate a solid. The solid was collected by filtration to give 12.3 g of 2-bromodibenzothiophene in 93.18% yield and ≥99% purity.

132-65-0
385 suppliers
$6.00/5g

22439-61-8
236 suppliers
$6.00/1g
Yield:22439-61-8 93.18%
Reaction Conditions:
with N-Bromosuccinimide in water;1,2-dichloro-ethane at 10; pH=3; for 12 h;pH-value;Reagent/catalyst;Temperature;Solvent;
Steps:
1-3; 1-2
9.2 g of dibenzothiophene was placed in a 500-ml three-necked round flask, and 150 ml of ethylene chloride was added. The dibenzothiophene was dissolved in a magnetic stirrer and the internal temperature of the reaction solution was adjusted to 10 ° C. 10.7 g of N-bromosuccinimide and 3 g of acid clay were added to the reaction solution and the reaction was started for 6 hours. At this time, the acid clay corresponds to about 32.6 wt% of dibenzothiophene. The acid clay was pH 3 (pH in 10% aqueous solution). After 6 hours of reaction, To the solution containing the reaction solution, N-bromosuccinimide and acid clay, 150 ml of a 5% aqueous sodium hydrogencarbonate solution was added and stirred for 1 hour to separate layers. Thereafter, the organic layer is washed twice with water, and then all of the solvent is distilled off to produce a solid. FIG. 3 is a graph showing the result of a solid produced by the above production method at a wavelength of 254 nm using HPLC. As shown in FIG. 3, The solid component was 96.71% by weight of the product, 2-bromodibenzothiophene, It can be confirmed that the by-product is composed of 2.21% by weight, the starting material is dibenzothiophene is 0.01% by weight and other substances. 300 m? of acetonitrile was added to the solid, and the dissolution temperature was raised to 82 ° C to dissolve the solution. The solution was cooled down to 30 ° C and cooled to remove the precipitated solid. The precipitated solid is a by-product 2,7-dibromodibenzothiophene.310 ml of acetonitrile in the filtrate was distilled off and then cooled to room temperature. The resulting solid was filtered to obtain 12.3 g (yield: 93.18%) of 2-bromodibenzothiophene having a purity of 99% or more.
References:
KR101906794,2018,B1 Location in patent:Paragraph 0045; 0065; 0090-0144

132-65-0
385 suppliers
$6.00/5g

22439-61-8
236 suppliers
$6.00/1g
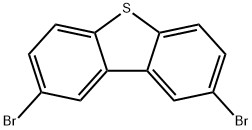
31574-87-5
209 suppliers
$7.00/250mg

62986-54-3
0 suppliers
inquiry

132-65-0
385 suppliers
$6.00/5g
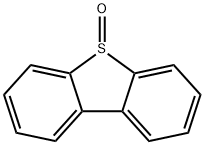
1013-23-6
79 suppliers
$74.00/100mg

22439-61-8
236 suppliers
$6.00/1g