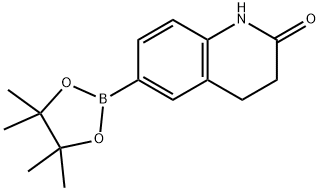
2-dioxaborolan-2-yl)quinolin-2(1H)-one synthesis
- Product Name:2-dioxaborolan-2-yl)quinolin-2(1H)-one
- CAS Number:400620-72-6
- Molecular formula:C15H20BNO3
- Molecular Weight:273.14
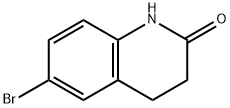
3279-90-1
161 suppliers
$10.00/1g

73183-34-3
593 suppliers
$6.00/5g

400620-72-6
58 suppliers
$112.00/100mg
Yield: 50%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 120;Inert atmosphere;
Steps:
S-16.1
Step 1 : Synthesis of 6-(4,4, 5, 5-tetramethyl-l, 3, 2-dioxaborolan-2-yl)-3,4- dihydroquinolin-2(lH)-one. To a solution of 6-bromo-3,4-dihydroquinolin-2(lH)-one (500 mg, 2.21 mmol, 1.0 equiv) in Dioxane (10 mL) was added 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(l,3,2- dioxaborolane) (561 mg, 2.21 mmol, 1.0 equiv), KOAc (650 mg, 6.63 mmol, 3.0 equiv) and the mixture purged with N2 gas for 10 min, followed by the addition of PdCk(dppf).DCM (90 mg, 0.1 1 mmol . 0.05 equiv). The resulting reaction mixture was heated at 120° C for overnight. After the completion of reaction (monitored by TLC & LCMS), the mixture was diluted with water (100 mL) and extracted with ethyl acetate (2 c 100 mL). Combined organic extracts were washed with water (50 mL * 2), dried over anhydrous NaiSOr and concentrated to obtain 6- (4,4,5,5-tetramethyi-L3,2-dioxaborolan-2-yl)-3,4-dihydroquinolin-2(lH)-one (300 mg, 50 % Yield) as an off white solid. LCMS 274.0 M 1 f j NMR (400 MHz, DMSG-ae) d 10.23 (s, 1H), 7.39 - 7.52 (m, 2H), 6.83 (d, J= 7.89 Hz 1H), 2.87 (t, J= 7.67 Hz, 2H), 2.39 - 2 47 (m, 2H), 1 .16 - 1.35 (m, 12H).
References:
PRAXIS BIOTECH LLC;PUJALA, Brahmam;PANPATIL, Dayanand;BERNALES, Sebastian;BELMAR, Sebastian;URETA DÍAZ, Gonzalo Andrés WO2020/132661, 2020, A2 Location in patent:Paragraph 0184
553-03-7
178 suppliers
$9.00/5g

400620-72-6
58 suppliers
$112.00/100mg

553-03-7
178 suppliers
$9.00/5g
2362-50-7
30 suppliers
inquiry

400620-72-6
58 suppliers
$112.00/100mg
92-85-3
159 suppliers
$6.00/5g