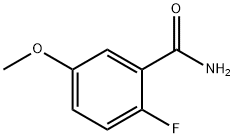
2-Fluoro-5-methoxybenzamide synthesis
- Product Name:2-Fluoro-5-methoxybenzamide
- CAS Number:400-92-0
- Molecular formula:C8H8FNO2
- Molecular Weight:169.15
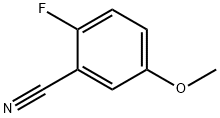
127667-01-0
188 suppliers
$10.00/1g

400-92-0
28 suppliers
$45.00/10mg
Yield:-
Reaction Conditions:
with dihydrogen peroxide;potassium carbonate in dimethyl sulfoxide at 20; for 0.5 h;
Steps:
36.A A. 2-Fluoro-5-methoxybenzamide, 36a
A. 2-Fluoro-5-methoxybenzamide, 36a To a solution of 2-fluoro-5-methoxybenzonitrile (2 g, 13.2 mmol) in DMSO (6.6 mL) was added H2O2 (1.6 mL) and K2CO3 (274 mg, 1.98 mmol). The resulting solution was stirred for 30 min at rt. The reaction was then quenched with NaHCO3 (satd, 30 mL). The resulting solution was extracted with EtOAc (50 mL), and the organic layers were combined, and concentrated under reduced pressure. The residue was purified by flash column chromatography (0-10% EtOAc/petroleum ether) on silica gel to obtain compound 36a as a white solid. 1H-NMR (400 MHz, DMSO-d6) (ppm): 7.66 - 7.70 (m, 2H), 7.04 - 7.23 (m, 3H), 3.77 (s, 3H).
References:
JANSSEN PHARMACEUTICA NV;HUANG, Hui;MEEGALLA, Sanath;PLAYER, Mark R. WO2017/27310, 2017, A1 Location in patent:Page/Page column 176
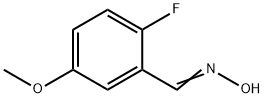
796120-91-7
1 suppliers
inquiry

400-92-0
28 suppliers
$45.00/10mg
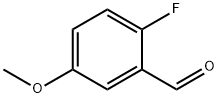
105728-90-3
179 suppliers
$12.00/1g

400-92-0
28 suppliers
$45.00/10mg