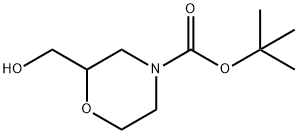
2-HYDROXYMETHYL-4-BOC-MORPHOLINE synthesis
- Product Name:2-HYDROXYMETHYL-4-BOC-MORPHOLINE
- CAS Number:135065-69-9
- Molecular formula:C10H19NO4
- Molecular Weight:217.26

24424-99-5
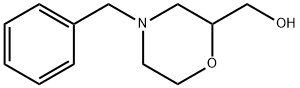
40987-24-4

135065-69-9
GENERAL STEPS: Ammonium formate (3.04 g, 48.2 mmol) and 10% palladium/activated charcoal (500 mg) were added to a methanolic solution (50 mL) of (4-benzylmorpholin-2-yl)methanol (1.00 g, 4.82 mmol). The reaction mixture was stirred at room temperature for 4 hours. After completion of the reaction, the mixture was filtered through diatomaceous earth and the filtrate was concentrated under reduced pressure. The resulting residue was dissolved in acetonitrile (20 mL) and cooled in an ice bath. To this solution, di-tert-butyl dicarbonate (1.58 g, 7.23 mmol) and triethylamine (1.35 mL, 9.64 mmol) were added sequentially and the reaction mixture was stirred at room temperature for 3 hours. At the end of the reaction, the solvent was evaporated under reduced pressure, water was added to the residue and extracted with ethyl acetate. The organic phases were combined, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluent: hexane/ethyl acetate=5:1→1:1) to afford the target compound tert-butyl 2-(hydroxymethyl)morpholine-4-carboxylate (907 mg, 87% yield) as a colorless oil.1H NMR (400 MHz, DMSO-d6) δ 1.40 (9H, s), 2.47-2.66 (1H, m), and 2.73-2.92 (1H, m), 3.25-3.45 (4H, m), 3.70 (1H, d, J=13.4 Hz), 3.79 (1H, dd, J=11.6, 2.4 Hz), 3.85 (1H, d, J=12.8 Hz), 4.77 (1H, t, J=5.5 Hz).
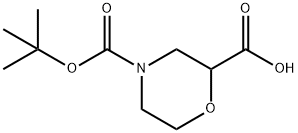
189321-66-2
144 suppliers
$5.00/250mg

135065-69-9
152 suppliers
$8.00/250mg
Yield:135065-69-9 97%
Reaction Conditions:
Stage #1:4-(tert-butoxycarbonyl)morpholine-2-carboxylic acid with borane-THF in tetrahydrofuran at 0 - 20; for 0.416667 h;
Stage #2: with methanol;acetic acid in tetrahydrofuran
Steps:
2.a
A solution of 4-{[(1 ,1-dimethylethyl)oxy]carbonyl}-2-morpholinecarboxylic acid (2.0 g, 21.6 mmol) in THF (45 mL) was cooled to 0 °C. A solution of borane (39 mL, 39.0 mmol, 1 M in THF) was added over 25 min via addition funnel. After warming to RT, the reaction was quenched by dropwise addition of methanol/acetic acid (18 mL, 9:1 v/v). The solvent was removed under reduced pressure and the residue partitioned between ethyl acetate and 1 N HCI. The aqueous layer was extracted with ethyl acetate and combined extracts were washed with water, 1 N NaOH, water, brine and dried over sodium sulfate. Removal of the solvent under reduced pressure afforded 1.83 g (97%) of the desired material. 1H NMR (400 MHz, CDCI3) δ ppm 1.49 (s, 9 H) 2.30 (br d, J=11.37 Hz, 1 H) 2.69 - 2.79 (m, J=9.51 , 6.41 , 3.28, 3.28 Hz, 1 H) 2.84 (ddd, J=13.77, 10.86, 3.16 Hz, 2 H) 3.27 - 3.38 (m, 1 H) 3.47 (br s, 1 H) 3.63 - 3.75 (m, 2 H) 4.10 - 4.19 (m, 1 H) 4.27 (br s, 1 H).
References:
SMITHKLINE BEECHAM CORPORATION WO2007/58850, 2007, A2 Location in patent:Page/Page column 43

24424-99-5
868 suppliers
$13.50/25G

40987-24-4
113 suppliers
$32.00/250mg

135065-69-9
152 suppliers
$8.00/250mg

24424-99-5
868 suppliers
$13.50/25G
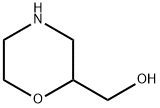
103003-01-6
111 suppliers
$45.00/50mg

135065-69-9
152 suppliers
$8.00/250mg
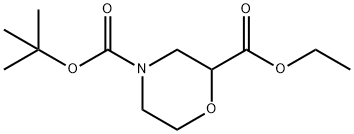
768371-16-0
70 suppliers
$18.00/250mg

135065-69-9
152 suppliers
$8.00/250mg
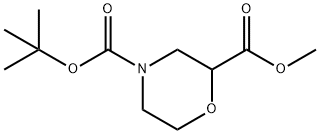
500789-41-3
61 suppliers
$21.00/100mg

135065-69-9
152 suppliers
$8.00/250mg