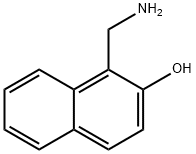
2-HYDROXYNAPHTHALEN-1-YLMETHYLAMINE synthesis
- Product Name:2-HYDROXYNAPHTHALEN-1-YLMETHYLAMINE
- CAS Number:5386-23-2
- Molecular formula:C11H11NO
- Molecular Weight:173.21
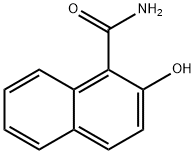
102880-70-6
0 suppliers
inquiry

5386-23-2
10 suppliers
inquiry
Yield:5386-23-2 23%
Reaction Conditions:
Stage #1: 2-hydroxy-1-naphthylamidwith borane-THF in tetrahydrofuran; for 2 h;Heating / reflux;
Stage #2: with hydrogenchloride in tetrahydrofuran;water; for 2 h;Heating / reflux;
Steps:
57
A solution of borane in tetrahydrofuran (19.23 mL of a 1M solution, 19.23 mmol) was added dropwise to a solution of the amide from preparation 52 (0.90 g, 4.81 mmol) in tetrahydrofuran (10 mL) and the reaction was then heated under reflux for 2 hours. The solution was cooled, treated with 6M hydrochloric acid (10 mL) and heated under reflux for a further 2 hours. The resulting suspension was cooled to room temperature and the pH was adjusted to pH 9 by addition of 0.88 ammonia and extracted with ethyl acetate (3x 50 mL). The combined organic solution was washed with brine (20 mL), dried over sodium sulfate and concentrated in vacuo. Purification of the residue by column chromatography on silica gel, eluting with dichloromethane : methanol : 0. 88 ammonia, 95: 5: 0.5 to 90: 10: 1, afforded the title compound as a pink solid in 23% yield, 0. 19 g. H NMR (400MHz, CD30D) 8 : 4.41 (2H, s), 7.07 (1H, d), 7. 23 (dd, 1H), 7.43 (1H, dd), 7.66 (1H, d), 7.72 (1H, d), 7.87 (1H, d); LRMS ESI m/z 174 [M+H] +
References:
WO2005/92840,2005,A1 Location in patent:Page/Page column 76-77
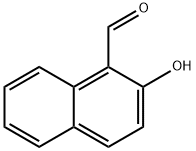
708-06-5
286 suppliers
$14.00/5g

5386-23-2
10 suppliers
inquiry
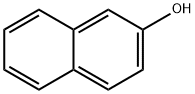
135-19-3
703 suppliers
$9.00/1g

5386-23-2
10 suppliers
inquiry
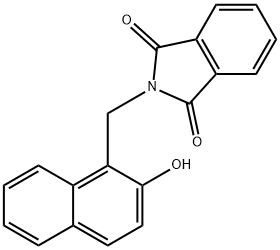
64489-92-5
11 suppliers
inquiry

5386-23-2
10 suppliers
inquiry