2-IODOBENZAMIDE synthesis
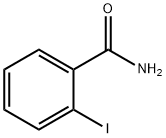
- Product Name:2-IODOBENZAMIDE
- CAS Number:3930-83-4
- Molecular formula:C7H6INO
- Molecular Weight:247.03
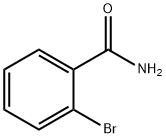
4001-73-4
179 suppliers
$8.00/1g

3930-83-4
111 suppliers
$9.00/250mg
Yield:-
Reaction Conditions:
with copper(l) iodide;sodium iodide;zinc(II) iodide;N,N`-dimethylethylenediamine in 1,4-dioxane at 120; under 1500.15 Torr; for 24 h;Autoclave;Inert atmosphere;Schlenk technique;Finkelstein Reaction;Reagent/catalyst;
Steps:
General procedure: (b) Methyl 2-bromo-5-hydroxybenzoate (4, 1.25 g, 5.41 mmol,1 equiv), Cu(I)I (103 mg, 541 mmol, 0.1 equiv), Zn(II)I2 (1.9 g,5.95 mmol, 1.1 equiv), and NaI (892 mg, 5.95 mmol, 1.1 equiv) were placed into a laboratory autoclave and flushed three times with argon. Then, 1,4-dioxane (50 mL) and N1,N2-dimethylethane-1,2-diamine (5, 136 μL, 1.08 mmol, 0.2 equiv) were added, and the autoclave was sealed. After 24 h at 120 °C (the pressure within the autoclave rises not above 2 bar) the solventwas evaporated under reduced pressure. The white residue wastaken up in water (100 mL) and extracted three times with EtOAc (100 mL). The combined organic phases were dried over sodium sulfate and concentrated to dryness under reduced pressure. The product was recrystallized from a mixture of cyclohexane and EtOAc (200 mL, 4:1 v/v). The title compound 7 was isolated in 66% yield by filtration as white solid (990 mg,3.56 mmol).
References:
Ueberschaar, Nico;Heine, Daniel;Hertweck, Christan [Synlett,2016,vol. 27,# 12,art. no. ST-2015-B0901-L,p. 1794 - 1797]
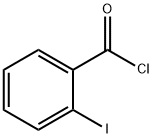
609-67-6
163 suppliers
$10.00/1g

3930-83-4
111 suppliers
$9.00/250mg
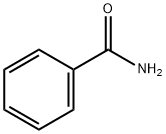
55-21-0
409 suppliers
$5.00/5 g

3930-83-4
111 suppliers
$9.00/250mg
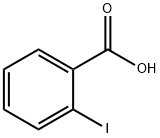
88-67-5
561 suppliers
$5.00/5g

3930-83-4
111 suppliers
$9.00/250mg
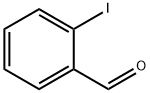
26260-02-6
242 suppliers
$8.00/250mg

3930-83-4
111 suppliers
$9.00/250mg