
2-IODOPHENYLHYDRAZINE HYDROCHLORIDE synthesis
- Product Name:2-IODOPHENYLHYDRAZINE HYDROCHLORIDE
- CAS Number:60481-34-7
- Molecular formula:C6H8ClIN2
- Molecular Weight:270.5
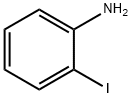
615-43-0
426 suppliers
$5.00/5g

60481-34-7
38 suppliers
$49.00/250mg
Yield:60481-34-7 96%
Reaction Conditions:
Stage #1: 2-iodophenylaminewith hydrogenchloride;sodium nitrite in water at 0; for 1.5 h;
Stage #2: with tin(ll) chloride in water at 0 - 20; for 14.5 h;
Steps:
7.A
Step A. 2-Iodoaniline (16 g, 73 mmol) was suspended in concentrated hydrochloric acid (100 mL), and then cooled to 0° C. in an ice bath. Sodium nitrite (6 g, 87.6 mmol) in water (25 mL) was added slowly to reaction mixture and then reaction allowed to stir at 0° C. for 1.5 hours. In a separate flask, tin (II) chloride (84.7 g, 182.5 mmol) was dissolved in concentrated hydrochloric acid (12 mL) and added slowly over 30 minutes to reaction mixture. The resulting suspension was allowed to warm to room temperature and stirred for 14 h. The solid was filtered off, and allowed to dry to afford 1-(2-iodophenyl)hydrazine hydrochloride (19 g, 96%). 1H NMR (CDCl3, 300 MHz) δ 7.82 (dd, 1H, J=1.1, 7.7 Hz), 7.39 (dt, 1H, 1.2, 7.7 Hz), 6.96 (dd, 1H, J=1.1, 8.1 Hz), 6.82 (dt, 1H, J=1.1, 7.5) ppm. MS (ApCI) 275 (M++CH3CN+H).
References:
US2005/239768,2005,A1 Location in patent:Page/Page column 12