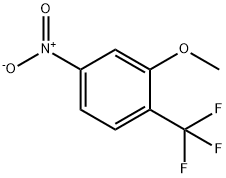
2-Methoxy-4-nitro-1-(trifluoromethyl)benzene synthesis
- Product Name:2-Methoxy-4-nitro-1-(trifluoromethyl)benzene
- CAS Number:453560-74-2
- Molecular formula:C8H6F3NO3
- Molecular Weight:221.13
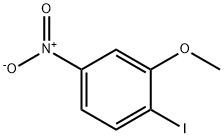
5458-84-4
127 suppliers
$9.00/1g
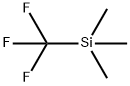
81290-20-2
417 suppliers
$13.00/1g

453560-74-2
56 suppliers
inquiry
Yield:453560-74-2 91%
Reaction Conditions:
with copper(l) iodide;cesium fluoride in sulfolane at 45; for 18 h;
Steps:
A.A61.1 Preparation of intermediate 444
A mixture of Cu l (6.80 g, 35.84 mmol ), CsF ( 14. 15 g, 93.18 mmol ) l -iodo-2-methoxy- 4-nitrobenzene ( 10 g, 35.84 mmol ) and sulfolane (20 ml ), was stirred rapidly at 45 °C. To this mixture was added trimethyl(trifluoromethyl)silane ( 13.25 g, 93. 1 mmol ) dropwise over 4 hours using a syringe pump, and the resulting mixture was stirred at 45°C for 1 8 hours. The mixture was diluted with ethyl acetate (500 mL ) and stirred with Celite for 5 min. The reaction mixture was filtered through a pad of Celite, diluted with ethyl acetate ( 500 mL ). The organic layer was washed with 10% NH4OH, 1.0 N HCl, brine, dried over Na2SC>4, filtered, and concentrated to give the crude product.The crude product was purified by chromatography (ethyl acetate/petroleum ether 0/1 to 1/4) to give intermediate 444 (8 g, 91% yield ) as a white solid.
References:
WO2017/32840,2017,A1 Location in patent:Page/Page column 226
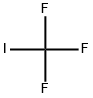
2314-97-8
186 suppliers
$40.00/25g

5458-84-4
127 suppliers
$9.00/1g

453560-74-2
56 suppliers
inquiry

5458-84-4
127 suppliers
$9.00/1g

453560-74-2
56 suppliers
inquiry