2'-METHYL[1,1'-BIPHENYL]-2-OL synthesis
- Product Name:2'-METHYL[1,1'-BIPHENYL]-2-OL
- CAS Number:77897-02-0
- Molecular formula:C13H12O
- Molecular Weight:184.23
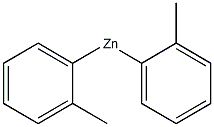
132-64-9
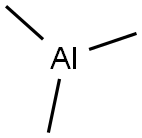
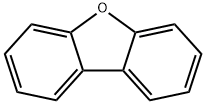
75-24-1
![2'-METHYL[1,1'-BIPHENYL]-2-OL](/StructureFile/ChemBookStructure8/GIF/CB7117343.gif)
77897-02-0
The general procedure for the synthesis of 2'-methyl-[1,1'-biphenyl]-2-ol from dibenzofuran and trimethylaluminum was as follows: to a 10 mL sample vial in a nitrogen-protected glove box, Ni(cod)2 (3.4 mg, 0.025 mmol), ICy-HBF4 (4.0 mg, 0.025 mmol), NaOtBu ( 48 mg, 0.50 mmol) and toluene (0.50 mL), followed by a Teflon-sealed screw cap. The mixture was stirred at room temperature for 3 minutes. Next, a toluene solution (0.50 mL) of aryl methyl ether (0.25 mmol) and AlMe3 (1.8 M toluene solution, 0.14 mL, 0.25 mmol) were added to the vial and resealed. The reaction mixture was stirred at 80 °C for 6 to 18 hours. After completion of the reaction, the mixture was cooled to room temperature and the crude product was treated with ethanol (EtOH). The reaction mixture was filtered through a silica gel pad and subsequently analyzed by GC. The filtrate was concentrated under vacuum and the residue obtained was purified by rapid column chromatography on silica gel.

132-64-9
361 suppliers
$5.00/10g

75-24-1
163 suppliers
$54.50/100ml
![2'-METHYL[1,1'-BIPHENYL]-2-OL](/StructureFile/ChemBookStructure8/GIF/CB7117343.gif)
77897-02-0
32 suppliers
$47.00/100mg
Yield:77897-02-0 60%
Reaction Conditions:
with bis(1,5-cyclooctadiene)nickel (0);sodium t-butanolate;1,3-bis(cyclohexyl)imidazolium tetrafluoroborate in toluene at 80; for 18 h;Sealed tube;Glovebox;Inert atmosphere;
Steps:
III. Typical Procedure for Ni/ICy-Catalyzed Methylation of Anisoles (Tables 1 and 2)
General procedure: Ni(cod)2 (3.4 mg, 0.025 mmol), ICy·HBF4 (4.0 mg, 0.025 mmol), NaOtBu (48 mg, 0.50 mmol) and toluene (0.50 mL) were added to a 10 mL-sample vial with a Teflon-sealed screwcap in a glovebox filled with nitrogen. The resulting mixture was stirred at room temperature for 3 min. An aryl methyl ether (0.25 mmol) in toluene (0.50 mL) and AlMe3 (1.8 M in toluene solution, 0.14 mL, 0.25 mmol) were then added to the vial and the screw cap was closed. The contents of the vial were stirred at 80 °C for 6 or 18 h. The reaction mixture was cooled to room temperature, and the crude mixture was then treated with EtOH. The resulting mixture was filtered through a pad of silica gel, and then analyzed by GC. The filtrate was concentrated in vacuo to give a residue, which was purified by flash column chromatography over silica gel.
References:
Morioka, Toshifumi;Nishizawa, Akihiro;Nakamura, Keisuke;Tobisu, Mamoru;Chatani, Naoto [Chemistry Letters,2015,vol. 44,# 12,p. 1729 - 1731] Location in patent:supporting information
95-56-7
303 suppliers
$9.00/5G
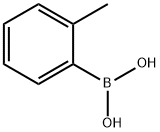
16419-60-6
379 suppliers
$9.00/1g
![2'-METHYL[1,1'-BIPHENYL]-2-OL](/StructureFile/ChemBookStructure8/GIF/CB7117343.gif)
77897-02-0
32 suppliers
$47.00/100mg
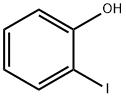
533-58-4
380 suppliers
$6.00/5g

16419-60-6
379 suppliers
$9.00/1g
![2'-METHYL[1,1'-BIPHENYL]-2-OL](/StructureFile/ChemBookStructure8/GIF/CB7117343.gif)
77897-02-0
32 suppliers
$47.00/100mg
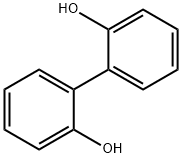
1806-29-7
317 suppliers
$10.00/5g
![2'-METHYL[1,1'-BIPHENYL]-2-OL](/StructureFile/ChemBookStructure8/GIF/CB7117343.gif)
77897-02-0
32 suppliers
$47.00/100mg