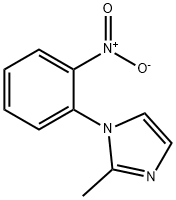
2-methyl-1-(2-nitrophenyl)-1H-imidazole synthesis
- Product Name:2-methyl-1-(2-nitrophenyl)-1H-imidazole
- CAS Number:26286-51-1
- Molecular formula:C10H9N3O2
- Molecular Weight:203.2
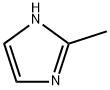
693-98-1
756 suppliers
$10.00/25g
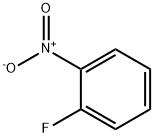
1493-27-2
511 suppliers
$5.00/10g

26286-51-1
19 suppliers
$96.00/250mg
Yield:26286-51-1 92%
Reaction Conditions:
with potassium carbonate in acetonitrile; for 24 h;Reflux;
Steps:
4.9. 2-methyl-1-(2-nitrophenyl)-1H-imidazole (4)
A mixture of 2-nitrofluorobenzene (3.74 mL, 0.035 mol), imidazole(2.62 g, 0.032 mol) and 10 g of K2CO3 in 50 mL of acetonitrilewas heated to reflux for 24 h. The solvent was removed undervacuum and the residuewas slurred in 50 mL dichloromethane and washed twice with distilled water. The organic layer was dried overNa2SO4 and the solvent was removed under vacuum. The compoundis obtained as a yellow solid (92% yield). C10H9N3O2. MW:203.20 g/mol. Mp 80-83 °C. 1H NMR δ (ppm, 300 MHz, CDCl3) 2.16(s, 3H, CH3), 6.86 (d, 1H, CH-N), 7.01 (d, 1H, CH-N), 7.39 (dd,1H, CH-C-N, J9 Hz), 7.62 (td, 1H, CH-C, J9 Hz), 7.72 (td, 1H,CHC-, J9 Hz), 7.99 (dd, 1H, CH-C-NO2, J9 Hz). 13C NMR δ (ppm,75 MHz, CDCl3) 13.03, 120.60, 125.34, 128.46, 130.12, 130.26, 131.00,133.94, 145.60, 146.27. MS (ESI +, QTof, m/z): 203.8 [M+H]+.
References:
Patinote, Cindy;Bou Karroum, Nour;Moarbess, Georges;Deleuze-Masquefa, Carine;Hadj-Kaddour, Kamel;Cuq, Pierre;Diab-Assaf, Mona;Kassab, Issam;Bonnet, Pierre-Antoine [European Journal of Medicinal Chemistry,2017,vol. 138,p. 909 - 919]