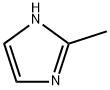
2-Methylimidazole synthesis
- Product Name:2-Methylimidazole
- CAS Number:693-98-1
- Molecular formula:C4H6N2
- Molecular Weight:82.1
It is obtained by eliminating dehydrogenation of 2-methylimidazoline. 2-methylimidazoline heated to melt (melting point 107 ℃), carefully add active nickel, raise the temperature to 200-210 ℃ reaction 2h. cool down to below 150 ℃, add water to dissolve, while hot pressure filtration, separation of active nickel, the filter Chemicalbook liquid concentrated to a temperature of 140 ℃ or more, put the material cooling that 2-methylimidazole. Use the method to produce purity of ≥ 98% of the product, 1t product consumption of ethylenediamine (95%) 1095kg, acetonitrile 975kg. better method is to use glyoxal and aldehyde as raw materials.
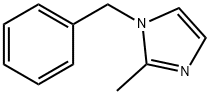
13750-62-4
204 suppliers
$23.00/25g

693-98-1
757 suppliers
$10.00/25g
Yield:693-98-1 97%
Reaction Conditions:
with triethylsilane;palladium 10% on activated carbon in tetrahydrofuran at 20; for 24 h;Inert atmosphere;
Steps:
General Procedure:
General procedure: A 16 mL vial with a PTFE/silicone septum and a nitrogen-bubbler line was charged with N-benzylbenzimidazole (208.1 mg, 1.0 mmol), Pd/C (10 wt %, dry powder, reduced) (20 mg) and THF (5 mL). The mixture was treated at rt with triethylsilane (320 μL, 2.0 mmol) and then stirred under nitrogen for 14 h at rt. The mixture was filtered through a 0.45 μM PTFE syringe filter and the filtrate was concentrated in vacuo. The residue was purified by column chromatography (0 to 10% MeOH/ dichloromethane) to afford benzimidazole as a colorless solid (117.6 mg, 0.996 mmol 99%). The reactions generally exhibit an induction period of 5 to 30 min as indicated by the initiation of gas release (i.e., bubbling) from the reaction mixture. The use of Pd/C on a dry matrix is imperative as wet Pd/C results in decreased yield or no reaction.
References:
Graham, Thomas H. [Tetrahedron Letters,2015,vol. 56,# 21,p. 2688 - 2690] Location in patent:supporting information
534-26-9
110 suppliers
$45.00/100mg

693-98-1
757 suppliers
$10.00/25g

131543-46-9
1 suppliers
inquiry

75-07-0
429 suppliers
$14.00/5mL

74-89-5
0 suppliers
$13.44/25ML
616-47-7
763 suppliers
$10.00/100g
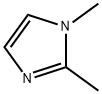
1739-84-0
421 suppliers
$6.00/25g

693-98-1
757 suppliers
$10.00/25g
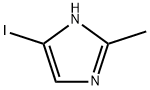
73746-45-9
102 suppliers
$12.00/250mg

693-98-1
757 suppliers
$10.00/25g