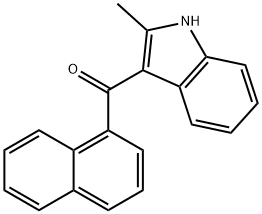
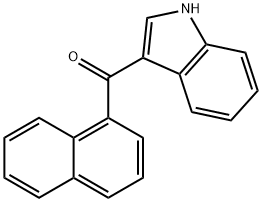
(2-Methyl-1H-indol-3-yl)-1-naphthalenylmethanone synthesis
- Product Name:(2-Methyl-1H-indol-3-yl)-1-naphthalenylmethanone
- CAS Number:80749-33-3
- Molecular formula:C20H15NO
- Molecular Weight:285.34
Yield:80749-33-3 70%
Reaction Conditions:
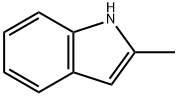
Stage #1: 2-methyl-1H-indolewith methylmagnesium bromide in diethyl ether at 0 - 20;
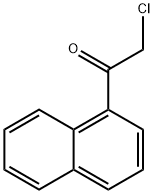
Stage #2: naphthalene-1-carbonic acid chloride in diethyl ether at 20; for 1.5 h;Reflux;
Stage #3: in diethyl ether;
Steps:
Synthesis of (2-Methyl-1H-indol-3-yl)-naphthalen-1-ylmethanone 15; 2-Methylindole (6.88 g, 52.46 mmol, 1 eq) was dissolved in ether (30 ml) and the solution was cooled to 0° C. MeMgBr (3M in ether, 62.95 ml, 62.95 mmol, 1.2 eq) was then added dropwise over 30 min and after the addition, the mixture was allowed to warm to ambient temperature. 1-Naphthoyl chloride (10 g, 52.46 mmol, 1 eq) in ether (15 ml) was added dropwise over 30 min and then the mixture was refluxed for 1 h, cooled and sat. aq. NH4Cl (200 ml) was added slowly to quench the reaction. The mixture was stirred until it was a pink slurry and the solids were then removed via filtration and were washed with water (50 ml). The solids were suspended in methanol (200 ml), a solution of NaOH (3 g) in water (100 ml) was added and the mixture was refluxed overnight. The solids were then filtered, washed with water (500 ml), washed with ether (250 ml) and were dried in vacuo. The solids were dissolved in DCM and were dry loaded onto silica and were then chromatographed in 1:1 CyH/EtOAc (Rf SM=0.9, Rf product=0.51, UV, KMnO4). This gave 10.847 g (70%) of the product as a pink solid. 1H-NMR (DMSO D6) 400 MHz: δ (ppm)=2.17 (3H, s, CH3), 3.34 (1H, s, NH), 6.94-6.99 (1H, m, NCCHCHCHCHC), 7.08-7.14 (1H, m, NCCHCHCHCHC), 7.25 (1H, br.d, J=8.0 Hz, NCCHCHCHCHC), 7.37 (1H, br.d, J=8.0 Hz, NCCHCHCHCHC), 7.44-7.58 (3H, m, CCHCHCHCCO, CCHCHCHCHCCCO), 7.61 (1H, dd, J=7.0 Hz, J=8.1 Hz, CCHCHCHCHCCCO), 7.83 (1H, br.d, J=8.3 Hz, CCHCHCHCCO), 8.03 (1H, br.d, J=8.2 Hz, CCHCHCHCHCCCO), 8.07 (1H, br.d, J=8.2 Hz, CCHCHCHCHCCCO). 13C-NMR (DMSO D6) 100 MHz: δ (ppm)=14.1 (CH3), 111.2 (CH), 113.7 (C), 120.1 (CH), 121.3 (CH), 122.0 (CH), 124.2 (CH), 124.7 (CH), 125.4 (CH), 126.2 (CH), 126.7 (CH), 126.9 (C), 128.2 (CH), 129.1 (CH), 129.3 (C), 133.1 (C), 134.9 (C), 140.5 (C), 145.7 (C), 191.9 (CO). IR Spectrum; evaporated film: v(cm-1)=3173, 1720, 1569, 1433, 1237, 1099, 1043. MS-ES (negative): 284.1 (M-H+). MS-ES (positive): 308.0 (M+Na+).
References:
US2011/39808,2011,A1 Location in patent:Page/Page column 47
95-20-5
367 suppliers
$5.00/10g
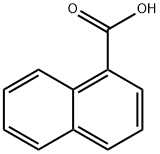
86-55-5
363 suppliers
$5.00/5g
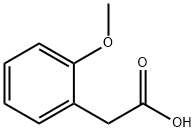
93-25-4
252 suppliers
$9.00/25g

80749-33-3
31 suppliers
$61.00/5mg

95-20-5
367 suppliers
$5.00/10g

86-55-5
363 suppliers
$5.00/5g
109555-87-5
109 suppliers
$61.00/5mg

80749-33-3
31 suppliers
$61.00/5mg
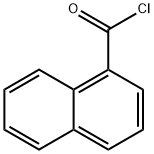
879-18-5
272 suppliers
$6.00/5g

80749-33-3
31 suppliers
$61.00/5mg