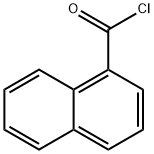
1-Naphthoyl chloride synthesis
- Product Name:1-Naphthoyl chloride
- CAS Number:879-18-5
- Molecular formula:C11H7ClO
- Molecular Weight:190.63
86-55-5

879-18-5
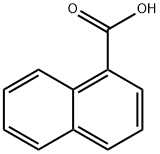
General method: Method A: 1-Naphthalenecarboxylic acid (5.8 mmol) was dissolved in dry toluene (40 mL) and thionyl chloride (8.0 mmol) was added. After refluxing the reaction for 2 hours, evaporate under reduced pressure to remove the solvent and excess thionyl chloride to give 1-naphthalenecarboxylic acid as a colorless liquid in quantitative yield. Upon cooling, the product formed colorless needle-like crystals with a melting point of 18-19°C (literature value 26°C [54]). 1-Naphthalenecarbonyl chloride (5.8 mmol), triethylamine (8.7 mmol) and the corresponding substituted aniline (5.8 mmol) were dissolved in anhydrous dichloromethane (30 mL), and the mixture was stirred at room temperature for 12 hours. After completion of the reaction, the solvent was removed by evaporation under reduced pressure, the solid residue was washed with 10% hydrochloric acid solution, the crude product was recrystallized with isopropanol and decolorized by the addition of activated carbon.

86-55-5
378 suppliers
$5.00/5g

879-18-5
280 suppliers
$6.00/5g
Yield:879-18-5 100%
Reaction Conditions:
with thionyl chloride in toluene; for 2 h;Reflux;
Steps:
General Procedure for Synthesis of Carboxamide Derivatives 1-8c
General procedure: Method A: Naphthalene-1-carboxylic acid (5.8 mmol) was dissolved in dry hot toluene (40 mL) and thionyl chloride (8.0 mmol) was added. After 2 h of refluxing, solvent and excessive thionyl chloride were evaporated under reduced pressure giving napthalene-1-carbonyl chloride as colourless liquid in quantitative yield. The product forms colourless needles after cooling. Mp. 18-19 °C (26 °C [54]). Naphthalene-1-carbonyl chloride (5.8 mmol), triethylamine (8.7 mmol) and corresponding substituted aniline (5.8 mmol) were dissolved in dry dichloromethane (30 mL) and the mixture was stirred for 12 h at ambient temperature. The solvent was evaporated under reduced pressure, the solid residue washed with 10% HCl, and the crude product was recrystallized from propan-2-ol with addition of active carbon.
References:
Gonec, Tomas;Kos, Jiri;Nevin, Eoghan;Govender, Rodney;Pesko, Matus;Tengler, Jan;Kushkevych, Ivan;Stastna, Vendula;Oravec, Michal;Kollar, Peter;O'mahony, Jim;Kralova, Katarina;Coffey, Aidan;Jampilek, Josef [Molecules,2014,vol. 19,# 7,p. 10386 - 10409]
90-14-2
294 suppliers
$19.00/5g
122-04-3
38 suppliers
$15.00/1g
636-98-6
38 suppliers
$17.00/5g

879-18-5
280 suppliers
$6.00/5g