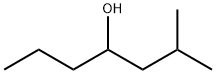
2-METHYL-4-HEPTANOL synthesis
- Product Name:2-METHYL-4-HEPTANOL
- CAS Number:21570-35-4
- Molecular formula:C8H18O
- Molecular Weight:130.23
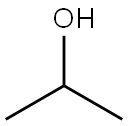
67-63-0
1641 suppliers
$16.00/25ML
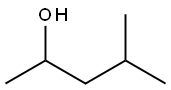
108-11-2
381 suppliers
$14.00/25mL

21570-35-4
17 suppliers
inquiry
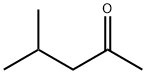
108-10-1
908 suppliers
$12.00/25ml
Yield: 21% , 19% , 9%
Reaction Conditions:
in o-xylene at 120; for 20 h;
Steps:
General procedure: We examined the scope of substrates for self-coupling of secondaryalcohols by using Ni/CeO2 (Ni = 1-3 wt%) reduced at 300 C(Table 4). Reaction of 2-octanol effectively proceeded at 130 Cfor 20 h to give the corresponding dimer products in high yield(entry 1). Self-coupling of other aliphatic liner alcohols proceededin good to moderate yield (entries 4-6). In the dimer products, 1(ketone) was mainly obtained and 2 (alcohol) was a minor products.Ni/CeO2 was also active for the self-coupling of cyclic alcohol, butthe main dimer product was not 1 but 2 (entry 7). Self-couplingof 2-propanol proceeded (entry 8) at much lower temperature(120 C) than those of the reported Cu-based catalysts [34-36](>200 C) and various coupling products, methyl isobutyl ketone(21%), 4-methyl-2-pentanol (19%), diisobutyl ketone (21%) and 2, 6-dimethyl-4-hexanol (9%), were obtained in comparable yields tothe previous studies. Note that the product of Aldol addition (nondehydratedproducts) was not observed by GC-MS. To the best ofour knowledge, the present system is the first heterogeneous catalyticsystem for self-coupling of various aliphatic alcohols underrelatively mild reaction conditions
References:
Shimura, Katsuya;Kon, Kenichi;Hakim Siddiki;Shimizu, Ken-Ichi [Applied Catalysis A: General,2013,vol. 462-463,p. 137 - 142]
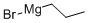
927-77-5
118 suppliers
$194.00/250g
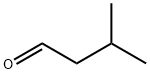
590-86-3
378 suppliers
$10.00/5g

21570-35-4
17 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

21570-35-4
17 suppliers
inquiry
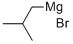
926-62-5
172 suppliers
$75.20/100ml

123-72-8
438 suppliers
$12.32/25ML

21570-35-4
17 suppliers
inquiry

10504-11-7
0 suppliers
inquiry

21570-35-4
17 suppliers
inquiry