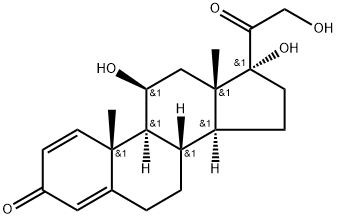
Prednisolone synthesis
- Product Name:Prednisolone
- CAS Number:50-24-8
- Molecular formula:C21H28O5
- Molecular Weight:360.45
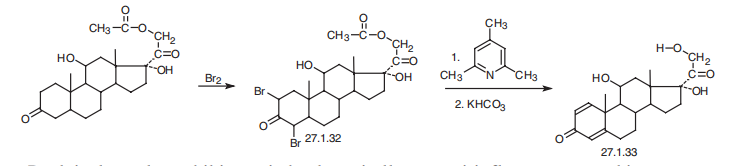
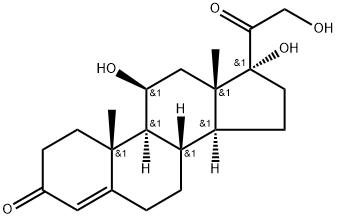
50-23-7
786 suppliers
$24.00/1g

50-24-8
626 suppliers
$16.39/100mg
Yield:50-24-8 97%
Reaction Conditions:
with Rhodococcus coprophilus DSM 43347 in dimethyl sulfoxide at 30; for 24 h;Green chemistry;regiospecific reaction;Reagent/catalyst;
Steps:
3.3. Semi-Preparative Biotransformation Protocol
General procedure: The biotransformations showing additional spots other than the starting material were repeated on a semipreparative scale (200 mL) in order to isolate the products. For each of the selected Rhodococcus strains, a loopful of bacterial cells cultured on PCA was transferred to 50 mL Erlenmeyer flasks containing 20 mL of sterile PCB medium and incubated at 30 °C and 110 rpm in an orbital shaker for 48 h. The whole culture was introduced into a 500 mL Erlenmeyer flask containing 200 mL of sterile PCB, and after 48 h incubation at 30 °C and 110 rpm, the substrate (0.2 g) in DMSO (2 mL) was added and the culture was maintained in the same conditions for a maximum of 72 h. All tests were carried out in triplicate for statistical significance. A blank sample was also incubated containing all the reagents but no bacteria. The reaction course was monitored by withdrawing samples (1 mL) every 24 h up to 72 h of reaction, and the semipreparative reactions were followed by TLC analysis and stopped when the higher conversion was reached. TLC analysis was performed using 1 ml of sample taken every 24 h from the flask in which the reaction was conducted. The sample was subsequently subjected to centrifugation, extraction with ethyl acetate, and immediately analyzed using TLC on silica gel using ethyl acetate as an eluent, following the same protocol used in the screening procedure. The cells were removed by centrifugation (5242 RCF, 20 min) and the supernatant was extracted with ethyl acetate (3 × 80 mL). The organic layer was dried over anhydrous Na2SO4, the solvent was evaporated, and the crude mixture was purified on a chromatographic column (silica gel, ethyl acetate/cyclohexane 50/50 as an eluent). The biotransformation yields were obtained by working out the percentage of the ratio between the moles of the product obtained compared to the moles of the administered substrate.
References:
Costa, Stefania;Fantin, Giancarlo;Semeraro, Bruno;Summa, Daniela;Zappaterra, Federico [Molecules,2020,vol. 25,# 9]
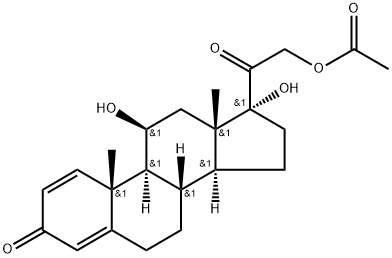
52-21-1
435 suppliers
$29.00/100mg

50-24-8
626 suppliers
$16.39/100mg

50-23-7
786 suppliers
$24.00/1g

50-24-8
626 suppliers
$16.39/100mg
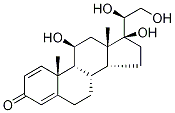
15847-24-2
24 suppliers
$60.00/1mg

98523-85-4
36 suppliers
$185.00/250mg

50-24-8
626 suppliers
$16.39/100mg
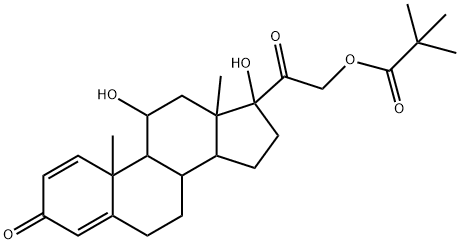
1107-99-9
34 suppliers
$135.00/100mg

50-24-8
626 suppliers
$16.39/100mg