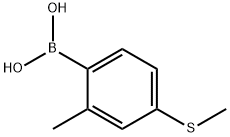
2-Methyl-4-methylthiophenylboronic acid synthesis
- Product Name:2-Methyl-4-methylthiophenylboronic acid
- CAS Number:444722-03-6
- Molecular formula:C8H11BO2S
- Molecular Weight:182.05
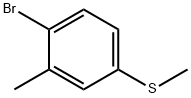
90532-02-8
36 suppliers
$60.00/100mg

444722-03-6
23 suppliers
$130.00/50mg
Yield:444722-03-6 52%
Reaction Conditions:
Stage #1: 1-bromo-2-methyl-4-methylsulfanylbenzenewith tert.-butyl lithium in diethyl ether at -78; for 0.283333 h;
Stage #2: with Trimethyl borate in diethyl ether at -78 - 20; for 0.283333 h;
Stage #3: with hydrogenchloride;ammonium chloridemore than 3 stages;
Steps:
15
Add 1M 3-methyl-phenylmagnesium bromide in THF (25 mL, 25 mmol) to-30°C diethyl ether (50 mL). Add dimethyl disulfide (1.8 mL, 20 mmol) to the reaction over 3 minutes allowing the reaction to warm to room temperature. Dilute the reaction in H20 (75 mL) and diethyl ether (50 mL). Separate and extract the cloudy aqueous layer with /diethyl ether (25 mL). Combine the organic layers and wash with H20 (25 mL). Dry the organic layer with Na2S04 (30 g), filter over Celite 501 (10 g) and concentrate in vacuo to give 3.0 g of crude material. Combine with another batch of crude title compound that yielded 6.0 g crude. Chromatograph the entire 9.0 g crude on a Si02 column in hexanes to give 7.2 g (70%) of 1-methyl-3-methylsulfanyl-benzene. Combine iron (25 mg, 0.45 mmol) and 1-methyl-3-methylsulfanyl-benzene (204 mg, 1.47 mmol) in dichloromethane (DCM, 2 mL). Cool the slurry to 3°C and add Br2 (74RL, 1.44 mmol) over 5 minutes. Stir at 3°C for 10 minutes and remove the external cooling bath. Stir at room temperature for 4 hours, then quench with 10% aqueous Na2S203 solution. Dilute the reaction with dichloromethane (10 mL) and separate. Wash the organic layer with brine, concentrate and chromatograph with dichloromethane in hexanes (0 to 5%) to give 125 mg of 1-bromo-2-methyl-4-methylsulfanyl-benzene (53%). Add l-bromo-2-methyl-4-methylsulfanyl-benzene (608 mg, 2.80 mmol) to diethyl ether (60 mL) and cool to-78°C under a nitrogen blanket. Add t-BuLi (3.4 mL, 5.78 mmol) over a 15 minute period, stir for 2 minutes, add trimethyl borate ((MeO) 3B, 340 RL, 2.99 mmol) over 2 minutes, stir for 15 minutes at-78°C and then let warm to room temperature. Quench the reaction with saturated aqueous NHLtCI (7 mL), stir for 15 minutes, add 1M aqueous HC1 (6 mL), stir for another 2 minutes and separate. Dry with Na2S04, filter (wash the drying agent with ethyl acetate (3 x 20 mL)), and concentrate. Chromatograph the crude material on a Si02 column with 20% ethyl acetate in hexanes to 5 % methanol in ethyl acetate to give 267 mg of the title compound (52%).
References:
WO2004/9086,2004,A1 Location in patent:Page/Page column 45