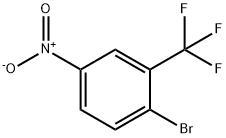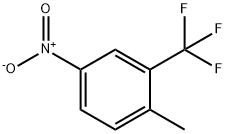
2-METHYL-5-NITROBENZOTRIFLUORIDE synthesis
- Product Name:2-METHYL-5-NITROBENZOTRIFLUORIDE
- CAS Number:89976-12-5
- Molecular formula:C8H6F3NO2
- Molecular Weight:205.13
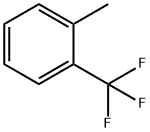
13630-19-8

89976-12-5
General procedure for the synthesis of 2-methyl-5-nitrobenzotrifluoride from o-toluotrifluoride: 50 g of 2-trifluoromethyltoluene and 250 g of 98% sulfuric acid were added to a 250 mL four-necked flask with stirring and cooled to 10°C. The reaction was carried out over a period of time. 50 g of 65% nitric acid was added slowly and dropwise over a period of about 2 hours to ensure complete reaction. Upon completion of the reaction, the reaction solution was poured into 200 g of crushed ice and layered with 150 mL of dichloromethane. The aqueous phase was extracted once more with 100 mL of dichloromethane and the organic phases were combined. The organic phase was washed sequentially with 80 mL of saturated sodium bicarbonate solution and 50 mL of water. Concentration to dryness gave 62 g of a light yellow oily product in 97% yield. The product did not need further purification and could be used directly in the next reaction.

13630-19-8
202 suppliers
$6.00/1g

89976-12-5
204 suppliers
$8.00/1g
Yield:89976-12-5 97%
Reaction Conditions:
with sulfuric acid;HNO3 at 10; for 2 h;Temperature;
Steps:
3 Example 3:
Synthesis of 4-nitro-2-trifluoromethyl toluene:Into a 250 ml four-necked flask, 50 g of 2-trifluoromethylbenzene and 250 g of 98% sulfuric acid were charged and stirred to cool down to 10 ° C. 50 g of 65% nitric acid was added dropwise over about 2 hours,To the reaction of raw materials completely, stop the reaction. The reaction solution was poured into 200 grams of crushed ice,Add 150 ml of dichloromethane, layer,The aqueous phase was extracted once more with 100 ml of dichloromethane, the organic phases were combined,And successively washed with 80 ml of saturated sodium bicarbonate and 50 ml of water,Concentration to dryness gave 62 g of a light yellow oil, yield: 97%. Without further purification of the next reaction.
References:
CN106316864,2017,A Location in patent:Paragraph 0049; 0050; 0056; 0057; 0063; 0064
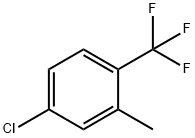
13630-22-3
16 suppliers
$248.00/1g

89976-12-5
204 suppliers
$8.00/1g
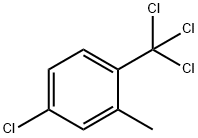
13630-15-4
1 suppliers
inquiry

89976-12-5
204 suppliers
$8.00/1g
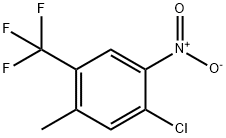
18018-35-4
23 suppliers
inquiry

89976-12-5
204 suppliers
$8.00/1g