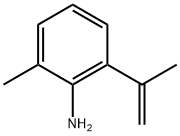
2-Methyl-6-(prop-1-en-2-yl)aniline synthesis
- Product Name:2-Methyl-6-(prop-1-en-2-yl)aniline
- CAS Number:107859-36-9
- Molecular formula:C10H13N
- Molecular Weight:147.22
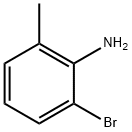
53848-17-2
145 suppliers
$45.00/1g
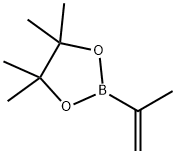
126726-62-3
177 suppliers
$38.50/1g

107859-36-9
6 suppliers
inquiry
Yield:107859-36-9 100%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;sodium carbonate in 1,4-dioxane;water;Inert atmosphere;Reflux;
Steps:
49.49a Step 49a: Step 49a: Preparation of 2-isopropyl-4,6-dimethylaniline (compound 0103-106)
Under the protection of nitrogen, the 2-bromo-6-methylaniline (1.86 g, 10.0 mmol, 1.0 equivalent), 4,4,5,5-tetramethyl-2-(prop-1-ene-2- Base)-1,3,2-dioxaborane (5.04 g, 30.0 mmol, 3.0 equivalents), sodium carbonate (2.12 g, 20.0 mmol, 2.0 equivalents) and Pd (dppf)Cl2 (0.73 g, 1.0 A mixture of millimoles, equivalent of 0.1) of dioxane/water = 5/1 (120 ml) was refluxed overnight. After cooling to room temperature, the mixture was filtered and concentrated in vacuo. The residue was purified by a chromatographic column (petroleum ether: ethyl acetate = 20:1) to obtain 2-methyl-6-(prop-1-en-2-yl)aniline as a colorless oil (1.5 g, yield: ~100.0%). MS (ES+) :/ζ = 148 (M+H) +.Under hydrogen conditions, the above-prepared 2-methyl-6-(prop-1-en-2-yl)aniline (1.47 g, 10 mmol, 1.0 equivalent) and palladium on carbon (0.5 g, 34% mass ratio) Ethyl acetate (50 ml) mixedThe mixture was stirred at room temperature overnight. The reaction solution was filtered, and the filtrate was concentrated under reduced pressure to obtain 2-isopropyl-4,6-dimethylaniline as a pale yellow oil (1.4 g, yield: 94%).
References:
CN112159405,2021,A Location in patent:Paragraph 0409-0410
59689-18-8
18 suppliers
inquiry

107859-36-9
6 suppliers
inquiry